Abstract
Lenvatinib is a multitargeted tyrosine kinase inhibitor approved for use in patients with iodine-131–refractory thyroid cancer. The common adverse events of lenvatinib include hypertension, proteinuria, fatigue, and diarrhea. To date, no report on Pneumocystis pneumonia (PCP) in patients receiving lenvatinib has been published. Here, we present a case of severe PCP that led to the death of a 79-year-old woman who was diagnosed with poorly differentiated thyroid cancer and received lenvatinib. The development of PCP should be considered when patients taking lenvatinib show clinical symptoms of pneumonia, and regular chest X-ray follow-up is needed for patients receiving lenvatinib.
Lenvatinib is a multitargeted tyrosine kinase inhibitor of the vascular endothelial growth factor receptor (VEGFR) 1–3, FGFRs 1–4, PDGFR α, RET, and KIT signaling network.1) Tyrosine kinase inhibitors play an important role in the treatment of progressive radioiodine refractory differentiated thyroid cancer (DTC). The common adverse events of lenvatinib include fatigue, hypertension, proteinuria, and diarrhea. In a recent phase 3 trial of lenvatinib, pneumonia was reported in approximately 2.3% of patients taking lenvatinib and was considered as a serious treatment-related adverse event, but the detailed clinical situation is not well known.2) Furthermore, prior to the current case, the occurrence of lenvatinib-induced Pneumocystis pneumonia (PCP) had not been reported. Here, we report a case of lenvatinib-induced PCP that led to the death of a patient with poorly differentiated thyroid cancer (PDTC).
A 78-year-old woman with a history hypothyroidism, hypertension, hyperlipidemia, and osteoporosis came to our clinic in April 2018 for a check-up of the thyroid function. We found a solid, round (5.6 cm in diameter), isoechoic, macro-calcified mass with smooth margin in the right portion of the thyroid, which had not significantly changed from the last examination. No cervical lymphadenopathy was apparent. The thyroid function test results were as follows: thyroid stimulating hormone (TSH) levels of 6.1 µU/mL (reference values: 0.4–5.0 µU/mL) and free T4 levels of 1.0 ng/dL (reference values: 0.8–1.9 ng/dL). We planned to do a follow-up examination of the thyroid function and ultrasonography (US) examination within 1 year.
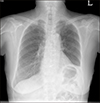
The patient went to another hospital because of an acutely developed neck discomfort 4 months later. A neck computed tomography (CT) scan showed a 6.8 cm heterogeneous attenuation mass at the right thyroid gland extending to the right internal jugular vein, brachiocephalic vein, and left inferior thyroidal vein; further, enlarged right hilar and subcarinal lymph nodes (LNs) suggested metastasis. The patient immediately underwent US-guided fine needle aspiration (FNA). The cytology results were categorized into the atypia of undetermined significance, but BRAFV600E mutation was detected. The patient then returned to our hospital for surgery. We performed total thyroidectomy with central node dissection and sternotomy for angioplasty because of tumor invasion of major veins. The pathology revealed a PDTC, 6 cm at its largest dimension, with gross extrathyroidal extension, intravascular extension, lymphatic invasion, and 10R LN metastasis. The pathologic staging was T4bN1bMx. After the surgery, the patient underwent radioactive iodine (RAI) therapy (100 mCi). The whole-body single-photon emission computed tomography (SPECT)/CT revealed a focal remnant thyroid tissue uptake in the left thyroid bed and multiple RAI non-avid metastatic LN and lung nodules. By using the eighth tumor node metastasis (TNM) staging system, we confirmed that the patient had an RAI-refractory PDTC with a stage 4 disease. A chest CT scan, performed 2 months after the surgery, showed significant progression of the lung and LN metastasis, and lenvatinib for palliative therapy was initiated. Her initial chest X-ray shows no active lung lesion (Fig. 1).
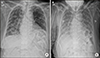
The patient was 153 cm tall and weighed 53 kg. Hence, the starting dose was 20 mg because the body surface area (BSA) was small (1.5 m2). During the initial period, we monitored the blood pressure and found that it increased on day 3. We therefore reduced the dose of lenvatinib to 14 mg and included amlodipine because the patient had been previously taking losartan. After a 4 week lenvatinib treatment, we prescribed methylprednisolone (4 mg twice daily for 7 days and then 4 mg once daily for 2 weeks) because the patient complained of skin urticaria with itching. After an 8 week lenvatinib treatment, the patient returned to the clinic with a recent onset of dyspnea and severe fatigue. Chest X-ray examination and chest CT showed newly developed multiple patchy opacities in both lungs, suggestive of PCP or drug-induced interstitial pneumonia, even though the metastatic lesions exhibited partial response to treatment according to the response evaluation criteria in solid tumors (RECIST) (Figs. 2, 3). The patient was immediately admitted to the general ward and lenvatinib was discontinued. The patient did not cough, nor did she have sputum, rhinorrhea or fever, and the patient only complained of modified Medical Research Council (mMRC) grade 3 dyspnea. The initial vital signs were blood pressure of 123/83 mmHg; pulse rate of 92/min; body temperature of 37.5℃; respiratory rate of 18/min; and peripheral capillary oxygen saturation of 95% at room air. We performed bronchoscopy and bronchoalveolar lavage (BAL) to confirm the etiology of pneumonia. The analysis of BAL fluid revealed 1200 RBC/µL and 209 WBC/µL (5% neutrophils, 65% lymphocytes, and 30% alveolar macrophages). Of note, the Pneumocystis jirovecii (P. jirovecii) DNA real-time polymerase chain reaction (PCR) CT was 26.34, suggesting a higher likelihood of Pneumocystis infection than of colonization. The Gram stain and bacterial culture, the AFB stain and culture, the fungus culture, and all respiratory virus (influenza, parainfluenza, adenovirus, and RSV virus) culture results were all negative. The serum titer of β-D-glucan, derived from the cell wall of several fungi, including Pneumocystis, was 221.3 pg/mL (reference value: <70 pg/mL). We checked the result of her serology test and found out it was human immunodeficiency virus (HIV) negative. We consulted a pulmonologist and an infection specialist regarding the BAL fluid analysis results and imaging findings. Because the BAL fluid analysis results and elevated serum β-D-glucan titer were typical for PCP, we started a therapeutic dose of sulfamethoxazole/trimethoprim to cover PCP and piperacillin/tazobactam with levofloxacin to cover the possibility of combined hospital-acquired bacterial pneumonia. However, the dyspnea worsened and the O2 supply was increased by replacing a nasal cannula 2 L/min with a high-flow nasal cannula (70% 40 L/min). Further, the chest X-ray image showed aggravation of the patchy opacities in both lungs (Fig. 4). We then included methylprednisolone (32 mg bid) as recommended for severe PCP, but the clinical course did not improve. The patient and her family decided against intubation and any further aggressive treatment. On day 16 of hospitalization, the patient died due to respiratory failure despite full antibiotic and steroid coverage.
PCP is a respiratory invasive infection caused by P. jirovecii, most frequently in immunocompromised patients. Patients who have PCP often present with low-grade fever, nonproductive cough, and progressive dyspnea. The diagnosis of PCP requires microscopic examination for Pneumocystis or detection of Pneumocystis nucleic acids by PCR in the sputum, bronchoalveolar fluid, or lung tissue. Trimethoprim/sulfamethoxazole is the most effective drug for treating severe PCP, and corticosteroids are of benefit in patients with PCP with hypoxemia who have underlying HIV.3) Host immunity is important in PCP infection. PCP usually affects individuals with cancer, acquired immune deficiency syndrome (AIDS), or solid organ or hematopoietic stem cell transplant, or those undergoing immunosuppressive therapies. The most significant risk factor for contracting PCP in HIV-uninfected patients is the use of glucocorticoids combined with an immunosuppressive therapy.4) In the current case report, the patient received steroid (methylprednisolone, 4 mg twice daily for 1 week and 4 mg once daily for 2 weeks) for skin urticaria, which can be a risk factor. However, typically, this steroid dose is not sufficient to cause PCP. Hence, we suggest that lenvatinib itself, acting via an immune-suppressive mechanism, may have been a risk factor. Since lenvatinib is a multitargeted tyrosine kinase inhibitor including the VEGF-R, and regulatory T cells are also known to express VEGFR-2.5) Regulatory T cell proliferative capacity can be inhibited in the presence of an anti-VEGFR-2 antibody and it could be related to the decreased immunity of the host.
Compared with PCP in HIV-infected patients, PCP in non-HIV-infected patients usually develops more rapidly and causes more severe oxygenation impairment, as in the current case.6) The mortality among non-HIV-infected patients with PCP is 30-60%, depending on the population at risk. The risk factors associated with increased mortality rate include old age, female sex, long time from onset of symptoms to diagnosis, respiratory failure, solid tumor, high lactate dehydrogenase levels, low serum albumin concentration, and bacterial and Aspergillus co-infection.7) In the current case, the age, sex, and solid cancer were all high-mortality risk factors and the patient died of respiratory failure.
Lenvatinib has been approved for use in RAI-refractory DTC; however, in the current case report, the patient was diagnosed with PDTC. Treatment of PDTC has not been standardized because of the rarity of the disease and the heterogeneity of the inclusion criteria.8) Surgery is the treatment of choice for PDTC, and the effectiveness of RAI therapy, external beam radiation therapy, or systemic chemotherapy in PDTC are not yet clear. In the current case, following a total thyroidectomy with LN dissection and angioplasty, we performed RAI, but the disease was refractory to RAI. Because the disease progressed within 3 months, we started lenvatinib treatment. The disease was partially responsive to lenvatinib, as determined by a follow-up chest CT scan, which suggested that lenvatinib might be effective against PDTC as well as against DTC.
One limitation of the current report is that the BAL analysis and CT findings can't rule out drug-induced interstitial pneumonia. Of the 28 currently approved tyrosine kinase inhibitors, 16 (57%) reportedly induce interstitial lung disease with variations in frequency and severity.9) A case report of interstitial pneumonia appeared during regorafenib administration and were exacerbated by subsequent lenvatinib therapy for advanced, unresectable HCC was introduced recently.10) Lenvatinib may inhibit the vascular endothelial growth factor (VEGF) pathway and contribute to progression of interstitial pneumonia. Compared with our case, the authors did not perform BAL and the respiratory symptoms improved after discontinuation of lenvatinib and started steroid treatment. However in our case, the result of BAL suggest PCP infection rather than colonization, and the clinical course worsened despite the discontinuation of lenvatinib and the use of corticosteroid. PCP rather than interstitial pneumonia may have been the determining factor in the patient's clinical course.
In the phase 3, randomized, double-blind, multicenter study (SELECT Clinical Trials), the rate of serious infection such as sepsis (0.8%), bacteremia (0.4%), and pneumonia necrotizing (0.4%) was not significantly higher than the placebo group.2) However, here we report a rare case of lenvatinib-induced PCP leading to death. Thus, we should be alert to the notion that lenvatinib may induce serious pneumonia and perform regular monitoring of respiratory symptoms and chest X-ray examinations of patients taking lenvatinib.
Figures and Tables
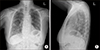 | Fig. 2Chest X-rays showing diffuse ground-glass opacities in the right lower lobe (A). Lateral view of the same-day X-ray image (B). |
References
1. Valerio L, Pieruzzi L, Giani C, Agate L, Bottici V, Lorusso L, et al. Targeted therapy in thyroid cancer: state of the art. Clin Oncol (R Coll Radiol). 2017; 29(5):316–324.

2. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372(7):621–630.

4. Truong J, Ashurst JV. Pneumocystis (Carinii) jiroveci pneumonia. StatPearls. Treasure Island (FL): StatPearls Publishing;2019.
5. French JD, Bible K, Spitzweg C, Haugen BR, Ryder M. Leveraging the immune system to treat advanced thyroid cancers. Lancet Diabetes Endocrinol. 2017; 5(6):469–481.

6. Tasaka S, Tokuda H. Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J Infect Chemother. 2012; 18(6):793–806.

7. Liu Y, Su L, Jiang SJ, Qu H. Risk factors for mortality from pneumocystis carinii pneumonia (PCP) in non-HIV patients: a meta-analysis. Oncotarget. 2017; 8(35):59729–59739.

8. Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly differentiated carcinoma of the thyroid gland: current status and future prospects. Thyroid. 2019; 29(3):311–321.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download