Abstract
Purpose
To correlate the value of contrast-enhanced ultrasound (CEUS) with prognostic factors of breast cancer.
Materials and Methods
24 breast cancer patients were evaluated with CEUS. As a quantitative analysis, the peak enhancement (PE), wash-in and wash-out area under curve (Wi-WoAUC), wash-in rate (WiR) and wash-out rate, rise time, fall time, mean transit time, time to peak, and wash-in perfusion index (WiPI) were measured. As a qualitative analysis, the enhancement patterns were evaluated. Pathologic prognostic factors, including histologic grade, hormonal receptors and Ki-67 proliferative index were assessed by immunohistochemistry. Correlation of quantitative and qualitative parameters of CEUS with prognostic factors was assessed.
Results
We found that the quantitative CEUS values (PE, WiWoAUC, and WiPI) of estrogen receptor (ER) positive breast cancer were higher than those of ER negative counterpart (allp < 0.05). Lower quantitative CEUS values (PE, WiWoAUC, WiR, and WiPI) were found in triple-negative cancer (TNC) than those of non-TNC (allp < 0.05). No CEUS parameter showed significant difference in distinguishing histologic grade (allp > 0.05).
Go to : 
References
2. Wan C, Du J, Fang H, Li F, Wang L. Evaluation of breast lesions by contrast enhanced ultrasound: qualitative and quantitative analysis.Eur J Radiol. 2012; 81:e444–e450.
3. Tuncbilek N, Unlu E, Karakas HM, Cakir B, Ozyilmaz F. Evaluation of tumor angiogenesis with contrast-enhanced dynamic magnetic resonance mammography. Breast J. 2003; 9:403–408.

4. Schroeder RJ, Bostanjoglo M, Rademaker J, Maeurer J, Felix R. Role of power Doppler techniques and ultrasound contrast enhancement in the differential diagnosis of focal breast lesions.Eur Rad/iiol. 2003; 13:68–79.
5. Xiao X, Ou B, Yang H, Wu H, Luo B. Breast contrast-enhanced ultrasound: is a scoring system feasible? A preliminary study in China. PLoS One. 2014; 9:e105517.

6. Li K, Su ZZ, Xu EJ, Ju JX, Meng XC, Zheng RQ. Improvement of ablative margins by the intraoperative use of CEUS-CT/MR image fusion in hepatocellular carcinoma. BMC Cancer. 2016; 16:277.

7. Wang YM, Fan W, Zhang K, Zhang L, Tan Z, Ma R. Comparison of transducers with different frequencies in breast contrast-enhanced ultrasound (CEUS) using SonoVue as contrast agent. Br J Radiol. 2016; 89:20151050.

8. Ricci P, Cantisani V, Ballesio L, Pagliara E, Sallusti E, Drudi FM, et al. Benign and malignant breast lesions: efficacy of real time contrast-enhanced ultrasound vs. magnetic resonance imaging. Ultraschall Med. 2007; 28:57–62.

9. Zhao H, Xu R, Ouyang Q, Chen L, Dong B, Huihua Y. Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions.Eur J Radiol. 2010; 73:288–293.
10. Hu Q, Wang XY, Zhu SY, Kang LK, Xiao YJ, Zheng HY. Meta-analysis of contrast-enhanced ultrasound for the differentiation of benign and malignant breast lesions.Acta Radiol. 2015; 56:25–33.
11. Wan CF, Du J, Fang H, Li FH, Zhu JS, Liu Q. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology. 2012; 262:450–459.

12. Liu H, Jiang Y, Dai Q, Zhu Q, Wang L, Lu J. Peripheral enhancement of breast cancers on contrast-enhanced ultrasound: correlation with microvessel density and vascular endothelial growth factor expression.Ultrasound Med Biol. 2014; 40:293–299.
13. Ji CL, Li XL, He YP, Li DD, Gu XG, Xu HX. Quantitative parameters of contrast-enhanced ultrasound in breast invasive ductal carcinoma: the correlation with pathological prognostic factors.Clin Hemorheol Microcirc. 2017; 66:333–345.
14. Nakata N, Ohta T, Nishioka M, Takeyama H, Toriumi Y, Kato K, et al. Optimization of region of interest drawing for quantitative analysis: differentiation between benign and malignant breast lesions on contrast-enhanced sonography. J Ultrasound Med. 2015; 34:1969–1976.
15. Masumoto N, Kadoya T, Amioka A, Kajitani K, Shigematsu H, Emi A, et al. Evaluation of malignancy grade of breast cancer using perflubutane-enhanced ultrasonography. Ultrasound Med Biol. 2016; 42:1049–1057.

16. Zhao YX, Liu S, Hu YB, Ge YY, Lv DM. Diagnostic and prognostic values of contrast-enhanced ultrasound in breast cancer: a retrospective study.Onco Targets Ther. 2017; 10:1123–1129.
17. Kim Y, Kim SH, Song BJ, Kang BJ, Yim KI, Lee A, et al. Early prediction of response to neoadjuvant chemotherapy using dynamic contrast-enhanced MRI and ultrasound in breast cancer.Korean J Radiol. 2018; 19:682–691.
18. Sinha S, Sinha N, Bandyopadhyay R, Mondal SK. Robinson's cytological grading on aspirates of breast carcinoma: correlation with Bloom Richardson's histological grading.J Cytol. 2009; 26:140–143.
19. Yi M, Huo L, Koenig KB, Mittendorf EA, Meric-Bernstam F, Kuerer HM, et al. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann Oncol. 2014; 25:1004–1011.

20. Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012; 30:729–734.

21. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Cl/iin Oncol. 2013; 31:3997–4013.

22. Penault-Llorca F, André F, Sagan C, Lacroix-Triki M, Denoux Y, Verriele V, et al. Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer.J Clin Oncol. 2009; 27:2809–2815.
23. Li X, Li Y, Zhu Y, Fu L, Liu P. Association between enhancement patterns and parameters of contrast-enhanced ultrasound and microvessel distribution in breast cancer. Oncol Lett. 2018; 15:5643–5649.

24. Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH, Pinder SE, et al. Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol. 2005; 18:26–35.

25. Zhao LX, Liu H, Wei Q, Xu G, Wu J, Xu HX, et al. Contrast-enhanced ultrasonography features of breast malignancies with different sizes: correlation with prognostic factors.BioMed Res Int. 2015; 2015:613831.
26. Koukourakis MI, Manolas C, Minopoulos G, Giatromanolaki A, Sivridis E. Angiogenesis relates to estrogen receptor negativity, c-erbB-2 overexpression and early relapse in node-negative ductal carcinoma of the breast. Int J Surg Pathol. 2003; 11:29–34.

27. Du J, Li FH, Fang H, Xia JG, Zhu CX. Microvascular architecture of breast lesions: evaluation with contrast-enhanced ultrasonographic micro flow imaging. J Ultrasound Med. 2008; 27:833–842. ; quiz 844.
28. Gown AM. Current issues in ER and HER2 testing by IHC in breast cancer.Mod Pathol. 2008; 21(Suppl 2):S8–S15.
29. Metz S, Daldrup-Unk HE, Richter T, Räth C, Ebert W, Settles M, et al. Detection and quantification of breast tumor necrosis with MR imaging: value of the necrosis-avid contrast agent Gadophrin-3.Acad Radiol. 2003; 10:484–490.
30. Poteca˘ T, Coma˘nescu M, Poteca˘ A, Cocosila C. The many faces of triple negative breast cancer.Chirurgia (Bucur). 2014; 109:471–479.
31. Jiang YX, Liu H, Liu JB, Zhu QL, Sun Q, Chang XY. Breast tumor size assessment: comparison of conventional ultrasound and contrast-enhanced ultrasound.Ultrasound Med Biol. 2007; 33:1873–1881.
Go to : 
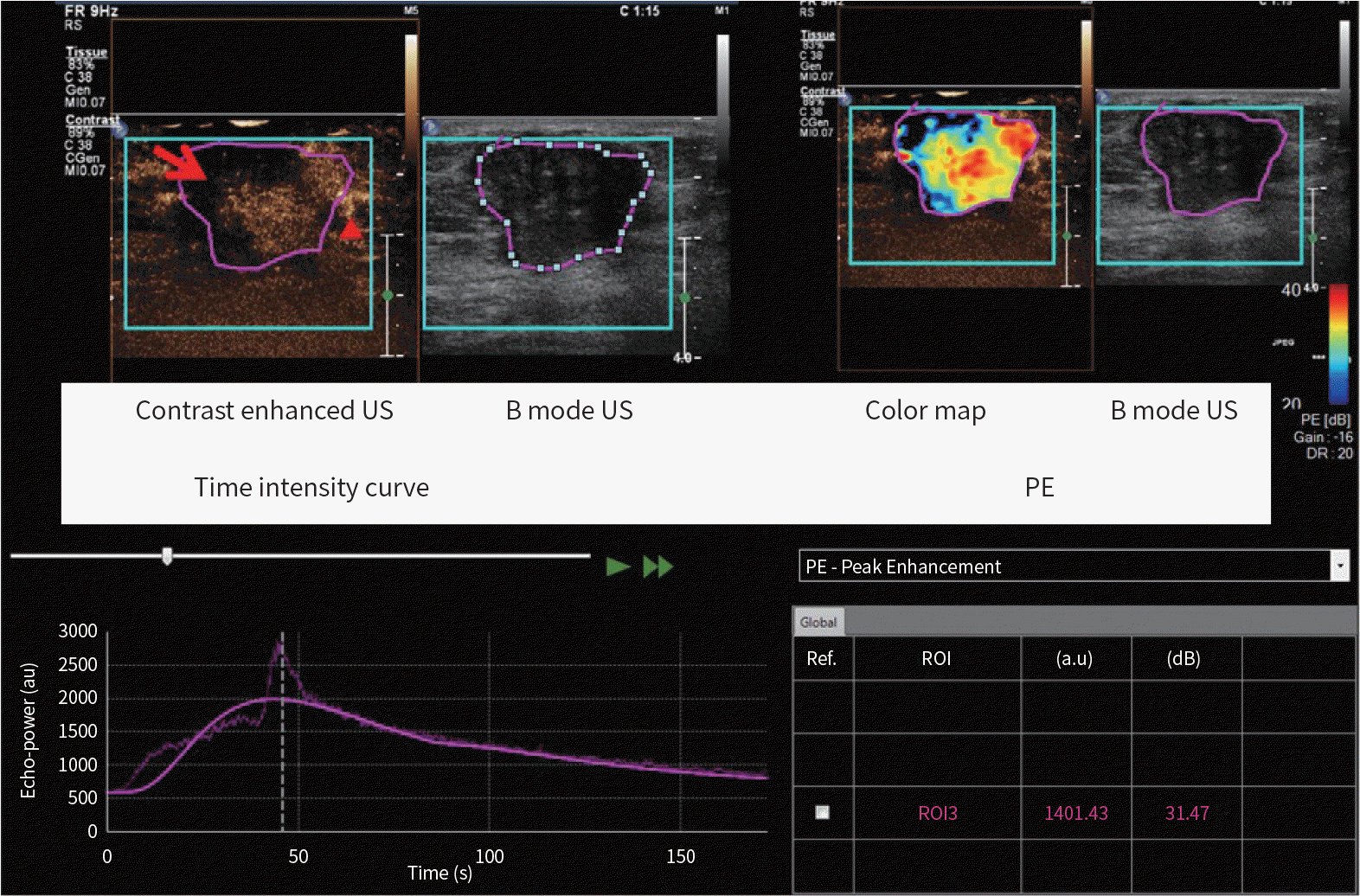 | Fig. 1.An example of imaging analysis with advanced US quantification software (VueBox®). B mode US image shows ROI (purple line) drawn manually covering the entire tumor. In all cases, the depth was set to 4 cm. Static image of contrast enhanced US at the PE shows heterogeneous enhancement, perfusion defect (red arrow) and crab-claw enhancement (red arrowhead) at the periphery. Color map with PE shows variable enhancement in the tumor. Time-intensity curve is shown. And the PE value is shown (about 1401 au). PE = peak enhancement, ROI = regions of interest, US = ultrasound |
 | Fig. 2.Quantitative time-to-intensity curve parameters. FT = fall time, mTTL = mean transit time, PE = peak enhancement, RT = rise time, TTP = time to peak, WiAUC = wash-in area under curve, WiR = wash-in rate, WiWoAUC = wash-in and wash-out area under the curve, WoAUC = wash-out area under curve, WoR = wash-out rate |
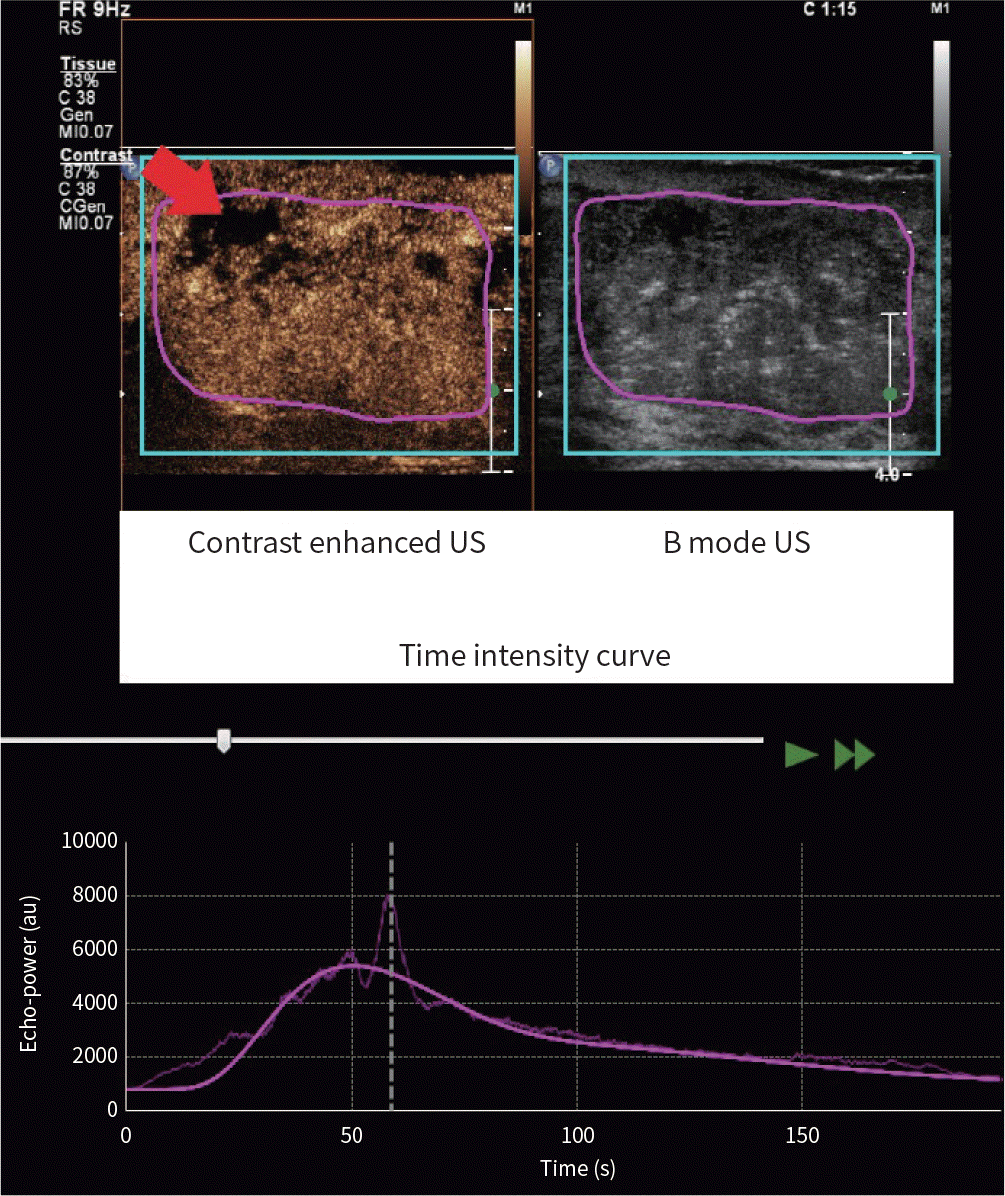 | Fig. 3.A 45-year-old woman presented with invasive ductal carcinoma, ER-positive type. The tumor showed a relatively high wash-in area under curve value (91280.77 au) of region of interest (purple circle). Other tumor characteristics were as follows: low histologic grade; ER positive; progesterone receptor positive; human epidermal growth factor receptor 2 negative; Ki-67 ≥ 14%; luminal subtype. Gray-scale breast US image shows an irregular hypoechoic mass. Contrast-enhanced US image shows heterogeneously hyper-enhanced mass with a local perfusion defect (red arrow). The scope of the lesion is larger than that shown by gray-scale US. Time-intensity curve shows peak intensity value (about 4627 au). ER = estrogen receptor, US = ultrasound |
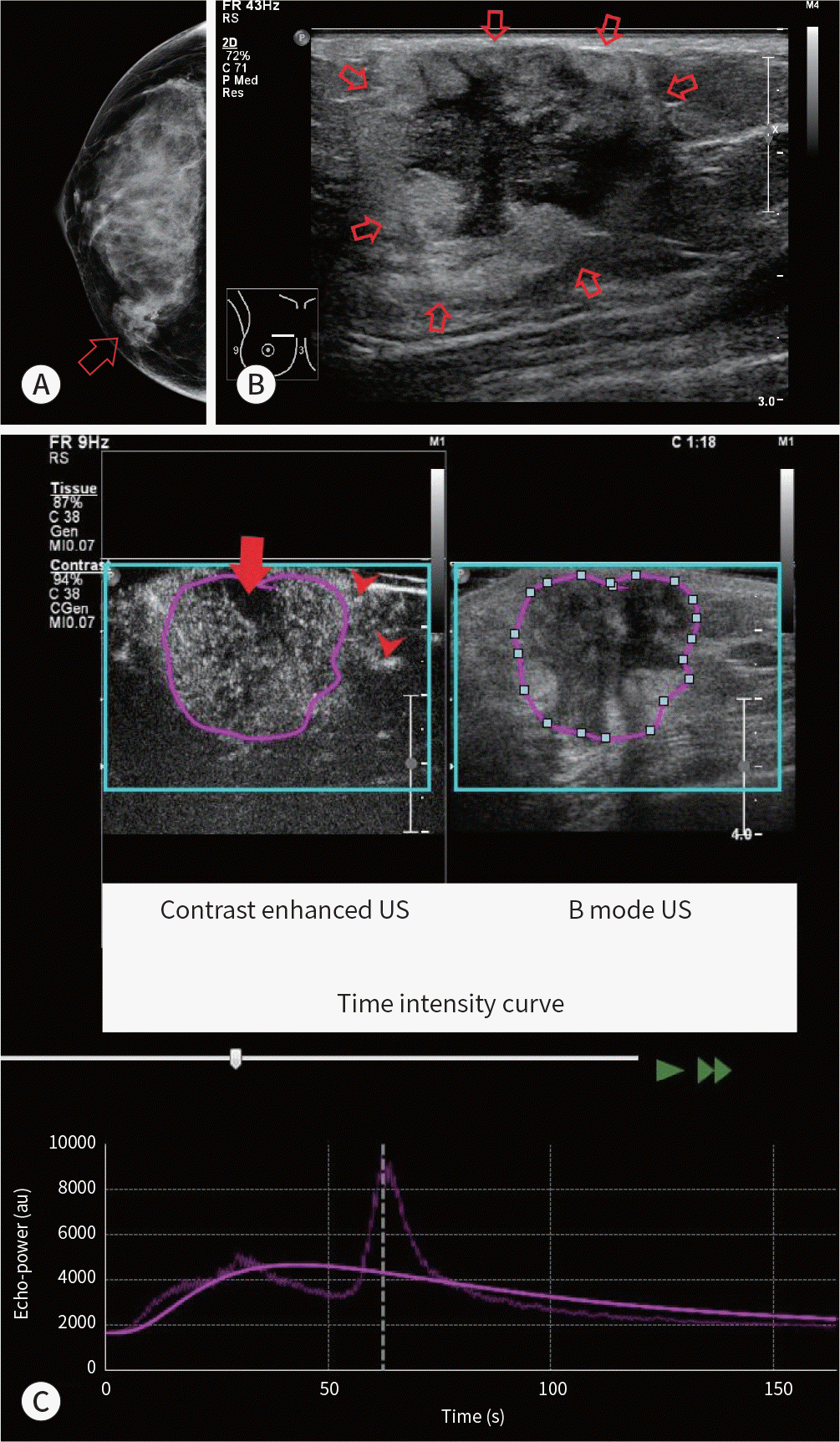 | Fig. 4.A 44-year-old woman presented with Rt breast cancer, ER-positive type. A. Mammography shows an indistinct oval equal density mass (arrow). B. Gray-scale breast US image shows an oval indistinct heterogeneous mass (central hypoechoic and peripheral hyperechoic) (arrows). C. Contrast-enhanced US image shows heterogeneously enhanced mass with a perfusion defect (red arrow) and crab-claw enhancement (red arrowheads) at the periphery. The scope of the lesion is slightly larger than it appears in the corresponding gray-scale US image. Time intensity curve shows peak intensity value (about 3164 au). The tumor showed a relatively high wash-in area under curve value (73005.78 au) of region of interest (purple circle). Other tumor characteristics were as follows: low histologic grade, ER positive, progesterone receptor positive, human epidermal growth factor receptor 2 negative, Ki-67 ≥ 14%, luminal subtype. ER = estrogen receptor, US = ultrasound |
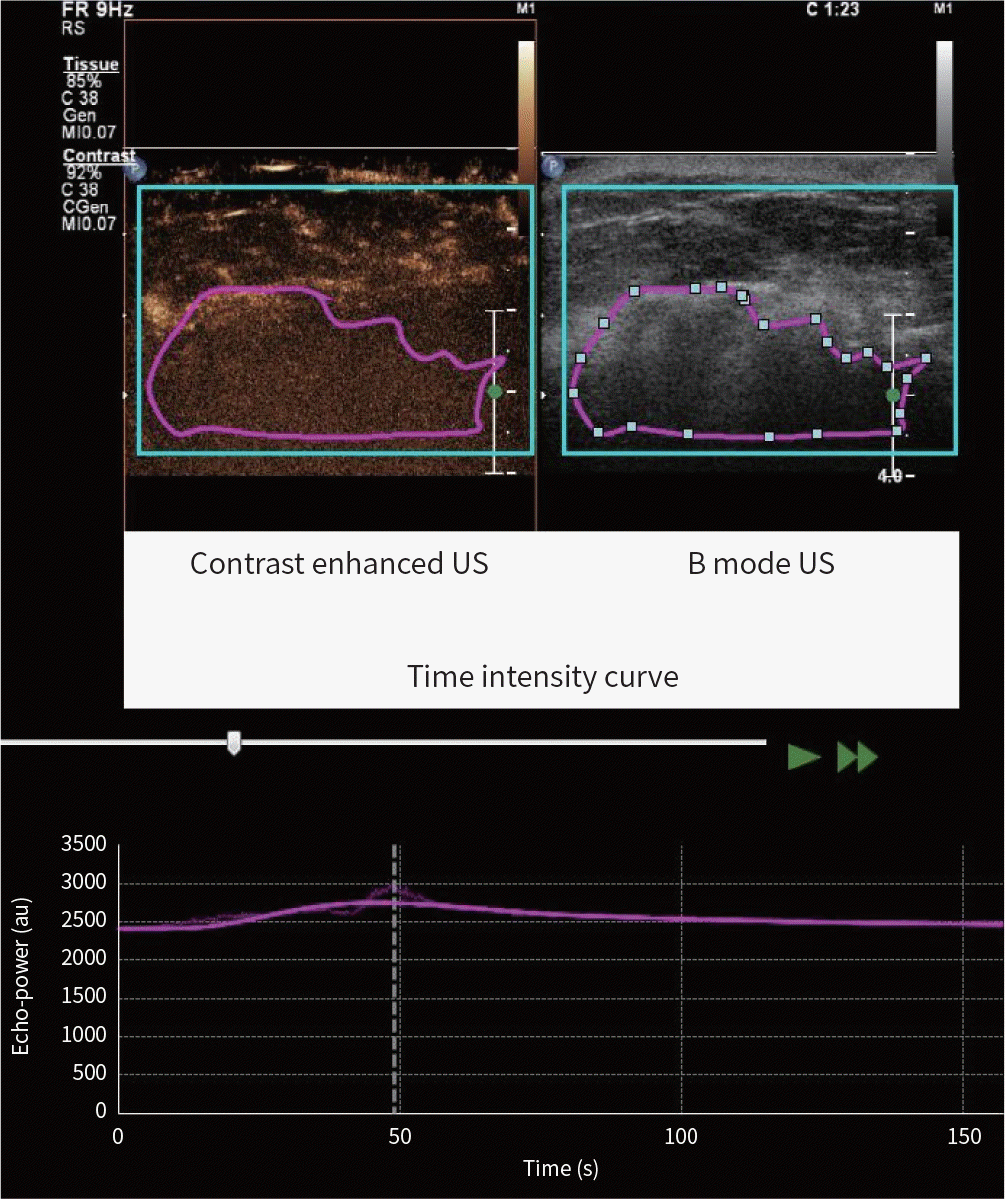 | Fig. 5.A 54-year-old woman presented with multi-centric breast cancer; invasive ductal carcinoma, triple negative subtype. The tumor showed a relatively low wash-in area under the curve, wash-out area under the curve, wash-in and wash-out area under the curve, wash-in perfusion index values of region of interest (purple circle). Other tumor characteristics were as follows: low histologic grade; estrogen receptor negative; progesterone receptor negative; human epidermal growth factor receptor 2 negative; Ki-67 ≥ 14%. Gray-scale breast US image shows an irregular indistinct hypoechoic mass. Contrast-enhanced US image shows relatively faint and heterogeneous enhancement. Time-intensity curve shows low peak intensity value (about 337 au). US = ultrasound |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download