Abstract
Various anomalies of the inferior vena cava (IVC) can arise from a failure in the normal embryogenic processes. Agenesis of the intrahepatic segment of the IVC with azygos continuation, which is caused by failure of formation of the right subcardinal–hepatic anastomosis, is a rare IVC anomaly. In this paper, we report a case of interrupted IVC with azygos vein continuation, combined with polysplenia, intestinal malrotation, and truncated pancreas, which was incidentally found on abdominal CT and thoracic aorta CT angiography.
초록
선천성 하대정맥의 변이는 태생기의 문합 형성의 이상으로 인해 발생한다. 하대정맥의 간부위 결손 및 홀정맥의 연장 역시 오른쪽 하주정맥이 원시 간의 모세혈관들과 연결되는 과정의 문제로 나타나는 드문 질환 중 하나이다. 저자들은 다중검출 컴퓨터단층촬영으로 성인에게서 우연히 발견된 하대정맥의 결손, 홀정맥의 연장, 췌장 끝부분의 소실 및 장회전 이상이 복합되어 있는 증례를 보고한다.
Aberrations in the complex embryogenesis of the inferior vena cava (IVC) can potentially result in anatomic variants. Interruption of the intrahepatic segment of the IVC with azygos continuation is a rare congenital anomaly. Conventionally, azygos vein dilatation results from the obstruction of the hepatic vein causing increased collateral venous flow, as observed in Budd-Chiari syndrome or portal hypertension (1); however, this can also occur in congenital anomalies. We report this rare, incidentally-found case of congenital anomaly of the intrahepatic segment of the IVC along with polysplenia, intestinal malrotation, and truncated pancreas.
A 24-year-old man was referred to our hospital with nonspecific abdominal pain. The patient's physical examination was unremarkable, and electrocardiography (ECG) showed a normal sinus rhythm. No abnormalities were detected from biochemical and serological tests. He had no history of surgery or trauma or any previously known systemic anomalies such as connective tissue disorders. The family history was nonspecific. The initial abdominal CT was performed on a 160-slice Aquilion PRIME CT scanner (Canon Medical Systems Corporation, Otawara-shi, Japan), after the use of 120 cc of iomeprol-400 (Iomeron®, Bracco U.K. Ltd., High Wycombe, United Kingdom) at a flow rate of 3 cc/s. The scanning protocol was as follows: pre-contrast scan (100 kVp, 160 mA), late arterial phase scan 80 s after bolus tracking (120 kVp, 106 mA), and delayed phase scan 180 s after bolus tracking (120 kVp, 121 mA). The axial and coronal images of abdominal CT in the delayed phase revealed several small sections of spleen along the greater curvature of the stomach. The pancreas showed a semiannular head with a tail defect in an axial image in the delayed phase (Fig. 1A).
The small bowel loop was located predominantly on the right side, while the large bowel loop was predominantly on the left in the delayed phase (Fig. 1B), with the ascending colon situated on the left side and the descending colon on the right. The abdominal CT in the late arterial phase demonstrated that the superior mesenteric vein was anterior to the superior mesenteric artery, contrary to their conventional positions (Fig. 1C). The location of the bowel loops and the reversal of the positions of superior mesenteric vessels were suggestive of intestinal malrotation. The liver, gallbladder, stomach, and duodenum were in their normal positions.
We performed further evaluation with thoracic aorta CT angiography because of the incidental discovery of a non-existent intrahepatic IVC. Thoracic aorta CT angiography with retrospective ECG gating was performed with a 320-slice Aquilion ONE GENESIS scanner (Canon Medical Systems Corporation). Patients' heartbeats were controlled using 50 mg atenolol (Tenormin®, AstraZeneca Pharmaceuticals, Wilmington, DE, USA) and 30 mg esmolol (Brevibloc®, Baxter Healthcare Corporation, Deerfield, MA, USA). Total 120 cc of iomeprol-400 was used at a flow rate of 5.5 cc/s. The scanning protocol was as follows: an arterial phase scan after the automated bolus triggering from the ascending aorta with an 8-s delay after contrast arrival is detected (100 kVp, 225 mA) and an additional delayed phase scan 200 s after bolus tracking (100 kVp, 225 mA). The axial scan in the delayed phase revealed that IVC was invisible above the level of the renal vein, and instead, both renal veins merged into the azygos vein (Fig. 1D) and acted as a functional IVC (Fig. 1E). The azygos vein then joined the superior vena cava (SVC) following its conventional path in the right paratracheal space, arching posteriorly over the right main bronchus at the root of the right lung (Fig. 1E). The hepatic veins drained directly into the right atrium in the coronal image taken in the delayed phase (Fig. 1F). The hemiazygos vein or vertebral venous plexus was not dilated, as found in Budd-Chiari syndrome. The heart was normal in shape and located in the left side of the chest. The course of the main bronchus on either side was normal, with the right main bronchus subdividing into three secondary bronchi and the left main bronchus into two secondary bronchi. The right main bronchus reached the root of the right lung at the level of the fifth thoracic vertebra, lying inferolateral and posterior to the right pulmonary artery.
A normal IVC is formed from the selective degeneration and anastomosis of three embryological veins: the supracardinal, postcardinal, and subcardinal veins. These veins develop into the four segments of the IVC in the 6th to 8th gestational weeks (2). The intrahepatic segment of the IVC is formed from the connection of the right proximal subcardinal vein with the capillaries of the primitive liver. The vitelline vein develops into the suprahepatic segment of the IVC. The prerenal division of the IVC occurs through the subcardinal vein. The renal segment is formed from suprasubcardinal anastomosis and postsubcardinal anastomosis. The postrenal segment is formed from the inferior portion of the right supracardinal vein.
In the case of azygos continuation, blood flows from the suprasubcardinal anastomosis through the dilated retrocrural azygos vein, which partially originates from the thoracic segment of the right supracardinal vein.
The renal portion of the IVC receives blood from both renal veins, passes posterior to the diaphragmatic crura, and enters the thorax as the azygos vein, through the aortic hiatus at the T12 vertebral level. The azygos vein then ascends in the posterior mediastinum and arches posteriorly over the right main bronchus at the root of the right lung, finally joining the SVC (3).
Polysplenia syndrome is a rare type of heterotaxy occurring in approximately 4 out of 1 million live births (4). In this condition, several small spleens are present in the left side of the upper abdomen, which accompanies various anomalies, including azygos or hemiazygos continuation, cardiac anomalies, intestinal malrotation, and gastrointestinal abnormalities (5).
The combination of failure of development, organ asymmetry, and situs ambiguus, results in bilateral left-sidedness, along with polysplenia. The genes involved in left-right laterality and heterotaxy syndrome include ZIC3, NODAL, LEFTY2, ACVR2B, CRYPTIC, CRELD-1, NKX2.5, and SHROOM3. The microdeletion of Xq26 and mutations in some of these genes have been identified in patients with heterotaxy (6).
Since the development of the dorsal pancreatic bud and spleen occur in the dorsal mesogastrium, pancreatic anomalies are often discovered in polysplenic patients (7). In the normal developmental process, the uncinate process and head of the pancreas originate from the ventral pancreatic bud, while the body and tail are derived from the dorsal one.
In this case, interruption of the infrahepatic IVC and azygos vein continuation occurred while the infrarenal IVC and iliac veins remained normal. This embryonic event was caused by the failure of formation of the right subcardinal–hepatic anastomosis, resulting in the atrophy of the right subcardinal vein.
Our case had no left-sidedness either in the chest or abdomen, including the liver, stomach, and gallbladder. The patient had azygos continuation with truncated pancreas and intestinal malrotation. This might go unnoticed and only be detected incidentally in radiological examination. Recent advances in CT technology have led to the incidental diagnoses of vascular and intestinal anomalies. Approximately 64 cases of polysplenia syndrome have been reported in the literature, based on previous CT and MRI findings (578910). However, to the best of our knowledge, only one case with a similar combination of these anomalies has been published (10). Abnormalities are not rare in polysplenia syndrome, but this combination of abdominal abnormalities is unusual. Knowledge of the diverse anomalies accompanying polysplenia syndrome is important both for surgeons and radiologists to avoid complications during surgical and interventional procedures such as left side appendectomy or femoral vein catheter advancement. Furthermore, some papers (35) showed that this malformation could cause venous insufficiency of the lower limbs with a potential thromboembolic disease, making periodic follow-up necessary.
In conclusion, to avoid the misinterpretation and misdiagnosis of the diverse anomalies accompanying polysplenia syndrome, it is crucial that radiologists recognize the anomalies appropriately and perform a comprehensive evaluation of the chest and abdomen.
Figures and Tables
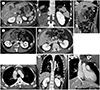 | Fig. 1A 24-year-old man with incidentally-found polysplenia, intestinal malrotation, and truncated pancreas, shown on abdominal CT and thoracic aorta CT angiography.
A. The axial and coronal images of abdominal CT in the delayed phase show that several spleens (arrowheads) are located on the left side of the upper abdomen along the greater curvature of the stomach; the pancreatic head is semiannular (arrow), and the tail shows a defect.
B. The coronal image of abdominal CT in the delayed phase demonstrates that the small bowel loop is predominantly located on the right side (arrow), while the large bowel loop is predominantly on the left (arrowhead).
C. The axial image of abdominal CT in the late arterial phase demonstrates that the superior mesenteric vein (arrow) is anterior to the superior mesenteric artery (arrowhead), contrary to their conventional positions.
D. An axial image of thoracic aorta CT angiography in the delayed phase showing that both renal veins (arrows) drain into the enlarged azygos vein instead of the inferior vena cava.
E. The axial and coronal image of thoracic aorta CT angiography in the delayed phase demonstrates an enlarged azygos vein (arrows) draining into the superior vena cava (arrowhead) at the level of the azygos vein arch.
F. A coronal image of thoracic aorta CT angiography in the delayed phase reveals that the hepatic veins (arrows) drain directly into the right atrium.
|
References
1. Simson IW. Membranous obstruction of the inferior vena cava and hepatocellular carcinoma in South Africa. Gastroenterology. 1982; 82:171–178.
2. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000; 20:639–652.
3. Demos TC, Posniak HV, Pierce KL, Olson MC, Muscato M. Venous anomalies of the thorax. AJR Am J Roentgenol. 2004; 182:1139–1150.
4. Tsutsumi R, Nagata Y, Enjoji A, Ohno Y, Kamito H, Kanematsu T. Situs ambiguous with gastric cancer: report of a case. Surg Today. 2007; 37:676–679.
5. Gayer G, Apter S, Jonas T, Amitai M, Zissin R, Sella T, et al. Polysplenia syndrome detected in adulthood: report of eight cases and review of the literature. Abdom Imaging. 1999; 24:178–184.
6. Shiraishi I, Ichikawa H. Human heterotaxy syndrome. -From molecular genetics to clinical features, management, and prognosis-. Circ J. 2012; 76:2066–2207.
7. Applegate KE, Goske MJ, Pierce G, Murphy D. Situs revisited: imaging of the heterotaxy syndrome. Radiographics. 1999; 19:837–852. discussion 853-854.
8. Rameshbabu CS, Gupta KK, Qasim M, Gupta OP. Heterotaxy polysplenia syndrome in an adult with unique vascular anomalies: case report with review of literature. J Radiol Case Rep. 2015; 9:22–37.
9. Soler R, Rodríguez E, Comesaña ML, Pombo F, Marini M. Agenesis of the dorsal pancreas with polysplenia syndrome: CT features. J Comput Assist Tomogr. 1992; 16:921–923.
10. Kobayashi H, Kawamoto S, Tamaki T, Konishi J, Togashi K. Polysplenia associated with semiannular pancreas. Eur Radiol. 2001; 11:1639–1641.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download