CASE REPORT
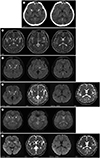
A 38-year-old man who had climbed a 5000-m-high mountain in China 5 days ago presented with general weakness and urinary incontinence. Because of these symptoms, he returned to Korea and was referred to our hospital. Despite being fully conscious and lucid, he was acutely indisposed. His cardiorespiratory parameters were within the normal limits; the blood pressure was 124/72 mm Hg, with oxygen saturation of 98% and blood glucose level of 105 mg/dL. The coordination between his upper and lower limbs was normal. He reported no contact with poisonous substances, such as carbon monoxide, 3,4-methylenedioxymethamphetamine (MDMA), cocaine, opiates, or cyanide. A brain CT scan revealed bilateral hypoattenuation in the globus pallidus and splenium of the corpus callosum (
Fig. 1A), without any evidence of recent intracranial hemorrhage. There were no abnormal attenuations indicative of venous thrombus on the CT scan. A 1.5 tesla brain MRI scan revealed high SI on an axial T2-weighted image (
Fig. 1B) and fluid-attenuated inversion recovery (FLAIR) sequences involving bilateral globus pallidus, posterior limb of the left internal capsule, and splenium of the corpus callosum (
Fig. 1C). The diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) maps showed restricted diffusion in bilateral globus pallidus and splenium of the corpus callosum (
Fig. 1D). On the gradient echo sequences (GRE), microbleeds with dark SIs were observed bilaterally in the globus pallidus. No abnormal dark SI was noted in the posterior limb of the left internal capsule and splenium of the corpus callosum (
Fig. 1E). There were no remarkable findings on T1-weighted images. Supportive treatment including intravenous dexamethasone and Cerebrolysin® (EVER Pharma; Unterach, Austria) was initiated. The patient was hospitalized for 1 week with complete functional recovery after 24 hours of admission. He was discharged on the seventh day, with advice to avoid further expeditions to high altitudes. The follow-up brain MRI after 5 weeks revealed complete resolution of the previously noted diffusion restriction in the splenium of the corpus callosum. However, encephalomalacia developed bilaterally in the globus pallidus (
Fig. 1F).
DISCUSSION
At high altitudes, hypoxia elicits responses that result in sustained vasodilatation, with impaired cerebral autoregulation, elevated capillary pressure, and leakage, resulting in edema formation (
3). Because of hypoxic cerebral vasodilatation, autoregulation may become transiently impaired, inducing a forceful increase in capillary hydrostatic pressure that promotes vasogenic edema, subsequent to the mechanical disruption of the blood–brain barrier and accumulation of vascular endothelial growth factor and reactive oxygen species (
4). Vasogenic edema preferentially spreads through the white matter, which is more sensitive to the imbalances in the demand and supply of cellular energy, resulting in fluid accumulation along the direction of orientation of the myelin fibers. The white matter has an orderly network of extracellular channels and offers little resistance to invasion by edematous fluid, while gray matter consists of tightly packed cellular structures (
5).
The characteristic finding of HACE on MRI is increased SI on T2-weighted images with restricted diffusion, particularly in the splenium of the corpus callosum, which is fully reversed on recovery (
1). Our case showed consistent radiologic findings with increased T2 and FLAIR SI and diffusion restriction in the splenium of the corpus callosum, along with full recovery.
The diffusion restriction observed in the splenium of the corpus callosum can be explained by its unique vascular permeability. Owing to the short arterioles in its anatomy and lack of pressure drop along the vessels to provide protection from decreased perfusion and ischemia (
5), the corpus callosum may be rendered more vulnerable to edema in the presence of hypoxic cerebral vasodilatation. Moreover, the neurons, astrocytes, and oligodendrocytes of the corpus callosum have a high density of receptors, including cytokine and glutamate receptors and other excitatory amino acids, toxins, and drug receptors (
6), which tends to cause cytotoxic edema in the corpus callosum. This suggests the coexistence of both vasogenic and cytotoxic edema in the corpus callosum before progressing to irreversibility.
Increased T2 and FLAIR SI were also seen in the posterior limb of the left internal capsule, without restricted diffusion. Typically, the posterior limb of the internal capsule is affected by hypoglycemic encephalopathy with high SI on T2 and with low SI on T1 and has restricted diffusion (
7). However, radiological examination of this patient showed a discrepancy between the T1-weighted image and the diffusion restriction. Furthermore, the patient did not have a history of diabetes, and the initial blood glucose level was within the normal range. The follow-up scan showed a full recovery of the posterior limb of the left internal capsule.
Moreover, in this case, additional increased T2 and FLAIR SI with restricted diffusion and microbleeds were noted bilaterally in the globus pallidus, with encephalomalacia on the follow-up scan. This is suggestive of the sequela of cytotoxic edema in the gray matter, which consists of tightly packed cellular structures, unlike the white matter (
5). Since this radiologic finding is atypical to HACE, a superimposed ischemic disorder should be considered. The globus pallidus is typically selectively spared, following a hypoxic-ischemic insult, in contrast to the caudate and putamen (
8). However, the reverse occurs in rare cases. The most common cause of bilateral globus pallidus necrosis is fatal carbon monoxide poisoning (
9). Additionally, fatalities associated with MDMA, cocaine, opiate, and cyanide poisoning have been reported (
9). As this patient denied having any contact with these poisonous substances, it could be attributed to altitude-related anoxic brain damage that is observed in ischemic-hypoxic encephalopathy. As the pathophysiology of HACE appears to involve reversible vasogenic and cytotoxic edema that progresses to microvascular disruption (
10), the microbleeds in the globus pallidus can be explained by HACE rather than any other condition.
In conclusion, the pathophysiological features of HACE are as follows: 1) reversible vasogenic edema in the posterior limb of the left internal capsule; 2) reversible vasogenic and cytotoxic edema in the corpus callosum; and 3) irreversible cytotoxic edema that progresses to microvascular disruption, causing bilateral microbleeds in the corpus callosum, which results in encephalomalacia. The initial DWI and ADC maps can be used as key indicators of treatment response and prognostic markers for future glial changes, except for atypical vascular permeability in the splenium of the corpus callosum. Detecting microbleeds with GRE imaging may also aid in the diagnosis, staging, and management of this serious condition.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download