CASE REPORT
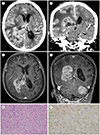
An 80-year-old woman had been experiencing headache for approximately one-and-a-half years. One month before visiting the authors' hospital, she developed cognitive impairment along with aggravation of the headache. She was bedridden at home for 10 days before visiting a local hospital where brain CT was performed. A brain tumor was suspected based on the CT scan, and she was referred to the authors' hospital. A neurological examination revealed drowsiness and decreased motor grade III in both lower extremities. The initial brain CT scan revealed a multilobulated, large mass with heterogenous enhancement in the right parietooccipital area (
Fig. 1A, B). The mass extended downward along the body, trigone, and occipital horn of the ipsilateral lateral ventricle, and measured 4.8 × 3.7 × 3.4 cm in size. Extensive peritumoral edema was observed in the right cerebral hemisphere, along with midline shifting to the left side. T1-weighted enhanced MRI revealed avid enhancement of the solid portion of the right lateral ventricular mass. Axial (
Fig. 1C) and coronal (
Fig. 1D) images revealed that the heterogeneously enhancing mass was located from the body to the occipital horn of the right lateral ventricle. On T2-weighted imaging, the solid portion exhibited isointensity and the inner portion exhibited necrotic change. A tentorial meningioma was found incidentally (
Fig. 1D). Considering the patient's age, and the shape and location of the tumor, the mass was initially suspected to be an intraventricular mass, such as choroid plexus carcinoma or a ventricular wall mass such as ependymoma; the possibility of GBM was also considered.
Partial surgical removal of the mass was performed under the guidance of a navigation system using the parietooccipital transcortical approach. Histopathological examination revealed glioblastoma, isocitrate dehydrogenase (IDH) wild type exhibiting nuclear polymorphism with several multinucleated giant cells and a high degree of cellularity (
Fig. 1E). Immunohistochemical tests were positive for glial fibrillary acidic protein (GFAP) (
Fig. 1F), epithelioid membrane antigen (EMA), and P53, with a Ki-67 labeling index of approximately 30%. In the gene mutation test, O-6-methylguanine-DNA methyltransferase (MGMT) methylation, 1p36 deletion, and IDH 1 R132 mutation were not detected, while a deletion at 19q13 was detected.
Postoperative CT revealed a residual mass in the body of the right lateral ventricle. Radiation therapy and chemotherapy (temozolomide) were deemed necessary. During the past year since surgery, the size of the tumor remains stable. After chemoradiotherapy, her intractable headache was slightly reduced.
DISCUSSION
GBM is the most aggressive and most common malignant primary brain tumor, accounting for approximately 15–20% of intracranial tumors and approximately 50% of gliomas in adults (
1). GBM can occur anywhere in the central nervous system, the predominant site being the cerebral cortex of the frontotemporal lobe (63%) (
2). However, GBMs of the intraventricular system are extremely rare. A PubMed search using the keywords “intraventricular GBM” or “intraventricular glioblastoma” retrieved a total of 31 cases. The most common site of intraventricular GBMs is the body of the lateral ventricle. One case involving the trigone and occipital horn of the lateral ventricle (
3), and another involving an area from the body to the trigone of the lateral ventricle (
4), have been reported; however, to date, there have been few—if any—case reports describing a GBM involving the body, trigone, and occipital horn of the lateral ventricle, as in our case. Cases involving most of the ventricles have been reported, but none involved the fourth ventricle. In our case, total resection of the mass was not possible because the GBM involved the body, trigone, and occipital horn of the lateral ventricle. However, the patient required decompression of the mass to reduce intractable headache. Therefore, concurrent chemo-radiation therapy was planned as a treatment option; however, it was not possible due to the poor general condition of the patient. The patient also carried gene mutations that are poor prognostic factors in cases of GBM. A previous study reported that that the 1p/19q co-deletion mutation is more conducive to chemotherapy (
5), but only the 19q deletion was detected in our patient. Among patients with intraventricular GBM, median survival is slightly is longer in those with the IDH mutation (
6); however, this was not detected in our patient. Therefore, the prognosis of our patient appears to be poor.
When a tumor develops in the ventricle, symptoms do not manifest until it grows and causes an obstructive hydrocephalus or a mass effect around it. In cases of GBM, the most common symptoms include headache, elevated intracranial pressure, and visual deficits. Changes in mental status, seizures, memory loss, focal motor deficits, and ataxia are less frequent symptoms. Psychiatric and memory disturbance can occur with forniceal involvement (
6). Our patient did not exhibit any specific signs or symptoms until the mass occupied more than one-half of the right lateral ventricle. If a GBM of the same size develops in the brain parenchyma, intracranial pressure will increase and signs and symptoms will appear much earlier. However, because intraventricular GBMs grow inward along the ventricle, the pressure increases relatively slowly and detection is delayed, as in our case.
Intraventricular tumors are generally divided into two types—primary and secondary—depending on the origin. Primary ventricular tumors are considered to occur along the ventricular wall or in structures within the ventricle. Tumors belonging to this group include choroid plexus carcinoma, choroid plexus papilloma, ependymoma, and meningioma. Secondary ventricular tumors exhibit transependymal development, originating from structures around the ventricle and invading the ventricle as the tumor grows. This group includes astrocytoma, choroid glioma, GBM, and mixed glial neuronal tumors. Based on the radiological findings in our case, we assumed that the transependymal development originated from the structure around the ventricle. It was presumed to originate from the subventricular zone (SVZ), among other structures. The SVZ is a space located in the lateral wall of the lateral ventricle and has several types of neural stem cells (NSCs) (
7). According to Doetsch et al. (
7), SVZ contains four distinct cell types: migrating neuroblasts (type A); SVZ astrocytes (type B1 and B2); immature precursors (type C); and ependymal cells. In vitro studies using mice have demonstrated that type B1 and B2 play a role given that NSCs can differentiate into regenerating and normal brain when types A and C are eliminated by antimitotic treatment (
7). According to Quiñones-Hinojosa et al., (
8) SVZ is composed of 4 layers: the ependymal layer (I); hypocellular layer (II); ribbon of cells (III); and the transition zone (IV). SVZ astrocytes are mainly distributed in layer III and, to some extent, in layer II, whereas displaced ependymal cells and neurons are distributed in several layers. In addition, many properties, such as self-renewal and multipotential capabilities, signaling pathways, behaviors and cell markers of NSCs in SVZ, were similar when brain tumor stem cells were extracted from malignant human tumors (
9). Therefore, NSCs in SVZ could be potential sources of brain tumors (
9).
When GBM occurs in the cerebral hemisphere, gadolinium-enhanced MRI usually reveals a large and peripheral, heterogeneously enhanced mass, often accompanied by necrotic changes or hemorrhage. However, MRI findings of intraventricular GBMs are rare; therefore, no characteristic radiological features have been established to date. Previously reported imaging findings of intraventricular GBMs range from well-defined margins and homogeneous enhancement (
6) to irregular shape and heterogeneous enhancement (
110). Our case was similar to that of GBMs in the brain parenchyma in that it demonstrated a multilobulated mass with heterogeneous peripheral enhancement and internal necrosis, except for the location of the tumor. In the case reported by Kim et al. (
1), a large mass with heterogeneous enhancement was observed, and the imaging findings of the tumor itself were similar to those in our case. However, unlike our case, tumors were formed in the medial wall of the lateral ventricle body and invaded the septum pellucidum and splenium of the corpus callosum, leading to obstructive hydrocephalus (
1). The characteristic imaging features of intraventricular GBM remain unclear; however, the possibility of GBM must be considered if an aggressive imaging finding is observed in the ventricular system.
GBM is a rapidly progressive tumor with a poor prognosis; therefore, prompt detection and appropriate treatment are important. Intraventricular GBM is very rare, but needs to be included in the differential diagnosis if clinical symptoms rapidly worsen and radiological findings suggest malignancy, such as the presence of a heterogeneously enhanced mass with extensive peritumoral edema.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download