1. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke.N Engl J Med. 1995; 333:1581–1587.
2. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a metaanalysis.Stroke. 2007; 38:967–973.
3. Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke.N Engl J Med. 2013; 368:893–903.
4. Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013; 368:904–913.

5. Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013; 368:914–923.

6. Khatri P, Hacke W, Fiehler J, Saver JL, Diener HC, Bendszus M, et al. State of acute endovascular therapy: report from the 12th thrombolysis, thrombectomy, and acute stroke therapy conference.Stroke. 2015; 46.
7. Berkhemer OA, Fransen PS, Beumer D, Van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20.
8. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection.N Engl J Med. 2015; 372:1009–1018.
9. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke.N Engl J Med. 2015; 372:1019–1030.
10. Jovin TG, Chamorro A, Cobo E, De Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; 372:2296–2306.

11. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke.N Engl J Med. 2015; 372:2285–2295.
12. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct.N Engl J Med. 2018; 378:11–21.
13. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378:708–718.

14. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018; 49:e46–e110.
15. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial.Lancet Neurol. 2016; 15:1138–1147.
16. Wardlaw JM, Seymour J, Cairns J, Keir S, Lewis S, Sandercock P. Immediate computed tomography scanning of acute stroke is cost-effective and improves quality of life. Stroke. 2004; 35:2477–2483.

17. Truwit CL, Barkovich AJ, Gean-Marton A, Hibri N, Norman D. Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction.Radiology. 1990; 176:801–806.
18. Marks MP, Holmgren EB, Fox AJ, Patel S, Von Kummer R, Froehlich J. Evaluation of early computed tomographic findings in acute ischemic stroke. Stroke. 1999; 30:389–392.

19. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000; 355:1670–1674.
20. Gupta AC, Schaefer PW, Chaudhry ZA, Leslie-Mazwi TM, Chandra RV, González RG, et al. Interobserver reliability of baseline noncontrast CT Alberta Stroke Program Early CT Score for intraarterial stroke treatment.
21. Bal S, Bhatia R, Menon BK, Shobha N, Puetz V, Dzialowski I, et al. Time dependence of reliability of noncontrast computed tomography in comparison to computed tomography angiography source image in acute ischemic stroke.Int J Stroke. 2015; 10:55–60.
22. Menon BK, Goyal M. Imaging paradigms in acute ischemic stroke: a pragmatic evidencebased approach. Radiology. 2015; 277:7–12.

23. Menon BK, Demchuk AM. Computed tomography angiography in the assessment of patients with stroke/TIA.Neurohospitalist. 2011; 1:187–199.
24. Menon BK, Campbell BC, Levi C, Goyal M. Role of imaging in current acute ischemic stroke workflow for en-dovascular therapy.Stroke. 2015; 46:1453–1461.
25. Nambiar V, Sohn SI, Almekhlafi MA, Chang HW, Mishra S, Qazi E, et al. CTA collateral status and response to.
26. Menon BK, D'Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a.
27. Liebeskind DS. Collateral circulation.Stroke. 2003; 34:2279–2284.
28. Cianfoni A, Colosimo C, Basile M, Wintermark M, Bonomo L. Brain perfusion CT: principles, technique and clinical applications. Radiol Med. 2007; 112:1225–1243.

29. Hunter GJ, Silvennoinen HM, Hamberg LM, Koroshetz WJ, Buonanno FS, Schwamm LH, et al. Whole-brain CT perfusion measurement of perfused cerebral blood volume in acute ischemic stroke: probability curve for regional infarction.Radiology. 2003; 227:725–730.
30. Menon BK, O'Brien B, Bivard A, Spratt NJ, Demchuk AM, Miteff F, et al. Assessment of leptomeningeal collat-erals using dynamic CT angiography in patients with acute ischemic stroke.J Cereb Blood Flow Metab. 2013; 33:365–371.
31. Goyal M, Menon BK, Derdeyn CP. Perfusion imaging in acute ischemic stroke: let us improve the science before changing clinical practice. Radiology. 2013; 266:1621.

32. Köhrmann M, Schellinger PD. Acute stroke triage to intravenous thrombolysis and other therapies with advanced CT or MR imaging: pro MR imaging.Radiology. 2009; 251:627–633.
33. Vilela P, Rowley HA. Brain ischemia: CT and MRI techniques in acute ischemic stroke.Eur J Radiol. 2017; 96.
34. Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4·5 h of symptom onset (PRE-FLAIR): a multicentre observational study.Lancet Neurol. 2011; 10:978–986.
35. Kinoshita T, Ogawa T, Kado H, Sasaki N, Okudera T. CT angiography in the evaluation of intracranial occlusive disease with collateral circulation: comparison with MR angiography.Clin Imaging. 2005; 29:303–306.
36. Wintermark M, Maeder P, Thiran JP, Schnyder P, Meuli R. Quantitative assessment of regional cerebral blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models.Eur Radiol. 2001; 11:1220–1230.
37. Wintermark M, Rowley HA, Lev MH. Acute stroke triage to intravenous thrombolysis and other therapies with advanced CT or MR imaging: pro CT.Radiology. 2009; 251:619–626.
38. Rudkin S, Cerejo R, Tayal A, Goldberg MF. Imaging of acute ischemic stroke. Emerg Radiol. 2018; 25:659–672.

39. Muir KW, Buchan A, Von Kummer R, Rother J, Baron JC. Imaging of acute stroke.Lancet Neurol. 2006; 5:755–768.
40. Rai AT, Seldon AE, Boo S, Link PS, Domico JR, Tarabishy AR, et al. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of en-dovascular stroke therapy in the USA.J Neuro/iinterv Surg. 2017; 9:722–726.
41. Lee JH, Han SJ, Yun YH, Choi HC, Jung S, Cho SJ, et al. Posterior circulation ischemic stroke in Korean population. Eur J Neurol. 2006; 13:742–748.

42. Kim JT, Park MS, Choi KH, Kim BJ, Han MK, Park TH, et al. Clinical outcomes of posterior versus anterior circulation infarction with low National Institutes of Health Stroke Scale Scores.Stroke. 2017; 48:55–62.
43. Pallesen LP, Lambrou D, Eskandari A, Barlinn J, Barlinn K, Reichmann H, et al. Perfusion computed tomography in posterior circulation stroke: predictors and prognostic implications of focal hypoperfusion.Eur J Neurol. 2018; 25:725–731.
44. De Lucas EM, Sánchez E, Gutiérrez A, Mandly AG, Ruiz E, Flórez AF, et al. CT protocol for acute stroke: tips and tricks for general radiologists.Radiographics. 2008; 28:1673–1687.
45. Van der Hoeven EJ, Dankbaar JW, Algra A, Vos JA, Niesten JM, Van Seeters T, et al. Additional diagnostic value of computed tomography perfusion for detection of acute ischemic stroke in the posterior circulation. .Stroke.
46. Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography.AJNR Am J Neuroradiol. 2005; 26:1012–1021.
47. Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke.
48. Wisco D, Uchino K, Saqqur M, Gebel JM, Aoki J, Alam S, et al. Addition of hyperacute MRI AIDS in patient selection, decreasing the use of endovascular stroke therapy. Stroke. 2014; 45:467–472.

49. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early man-agement of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44:870–947.
50. Sauser K, Burke JF, Levine DA, Scott PA, Meurer WJ. Time to brain imaging in acute stroke is improving: secondary analysis of the INSTINCT trial. Stroke. 2014; 45:287–289.
51. Sauser K, Levine DA, Nickles AV, Reeves MJ. Hospital variation in thrombolysis times among patients with acute ischemic stroke: the contributions of door-to-imaging time and imaging-to-needle time.JAMA Neurol. 2014; 71:1155–1161.
52. Wang H, Thevathasan A, Dowling R, Bush S, Mitchell P, Yan B. Streamlining workflow for endovascular mechanical thrombectomy: lessons learned from a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2017; 26:1655–1662.

53. Zaidi SF, Shawver J, Espinosa Morales A, Salahuddin H, Tietjen G, Lindstrom D, et al. Stroke care: initial data.
54. Shah S, Luby M, Poole K, Morella T, Keller E, Benson RT, et al. Screening with MRI for accurate and rapid stroke.
55. Li B, Li H, Dong L, Huang G. Fast carotid artery MR angiography with compressed sensing based three-dimensional time-of-flight sequence.Magn Reson Imaging. 2017; 43:129–135.
56. Wu EL, Kuo LW, Wu FH, Hsu CF, Hsieh CW, Chen JH, et al. Ultra-fast brain MR imaging using simultaneous multislice acquisition (SMA) technique. Conf Proc IEEE Eng Med Biol Soc. 2007; 2007:2618–2621.

57. Baek HJ. Two-minute MR ultra-fast neuro protocol. Available at.https://www.gehealthcare.co.uk/-/media/1386100484c8464bb08bd49f821b5cdb.pdf/. Published 2017. Accessed Jun 30,. 2018.
58. Nael K, Khan R, Choudhary G, Meshksar A, Villablanca P, Tay J, et al. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: pushing the boundaries.Stroke. 2014; 45:1985–1991.
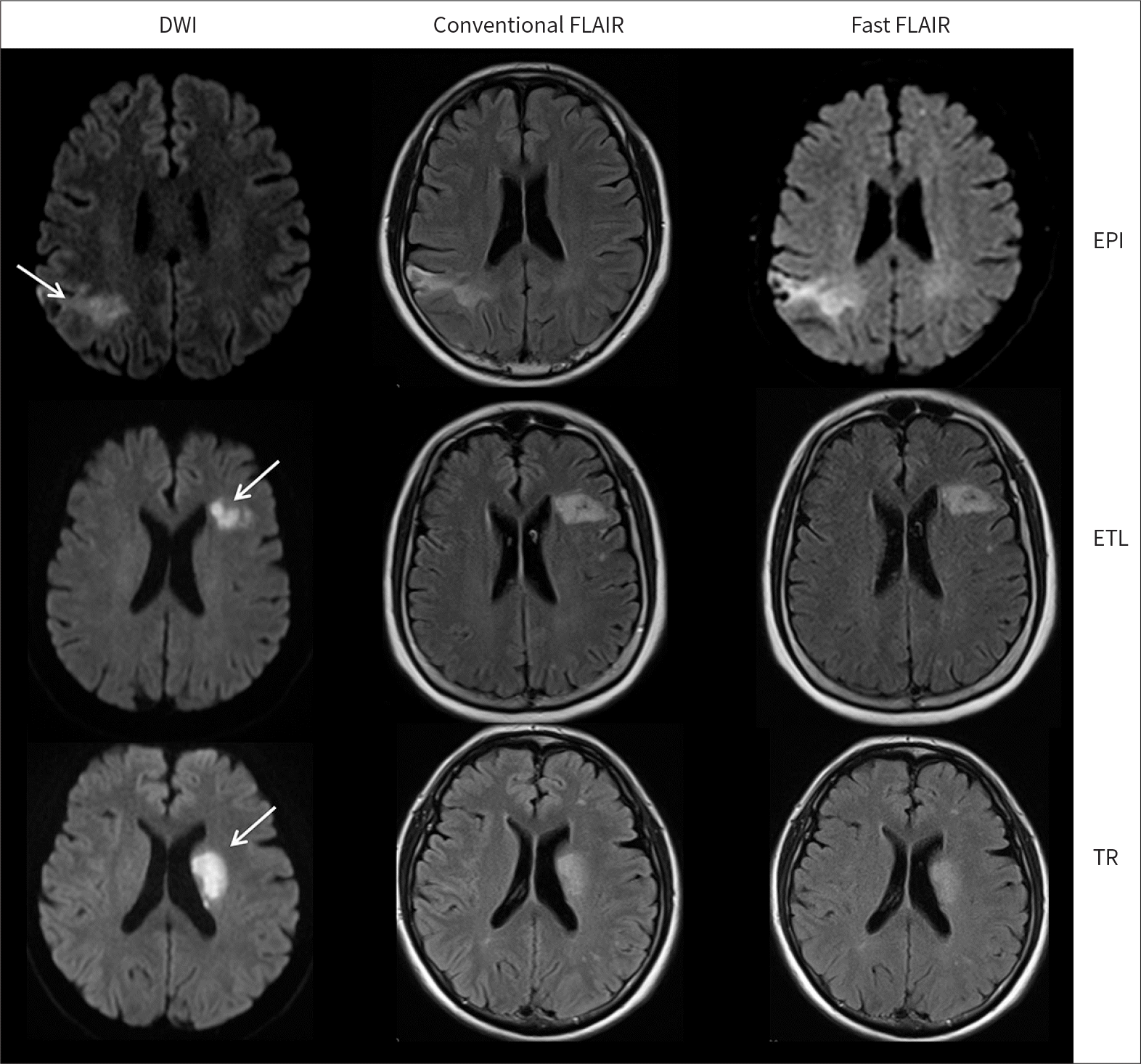

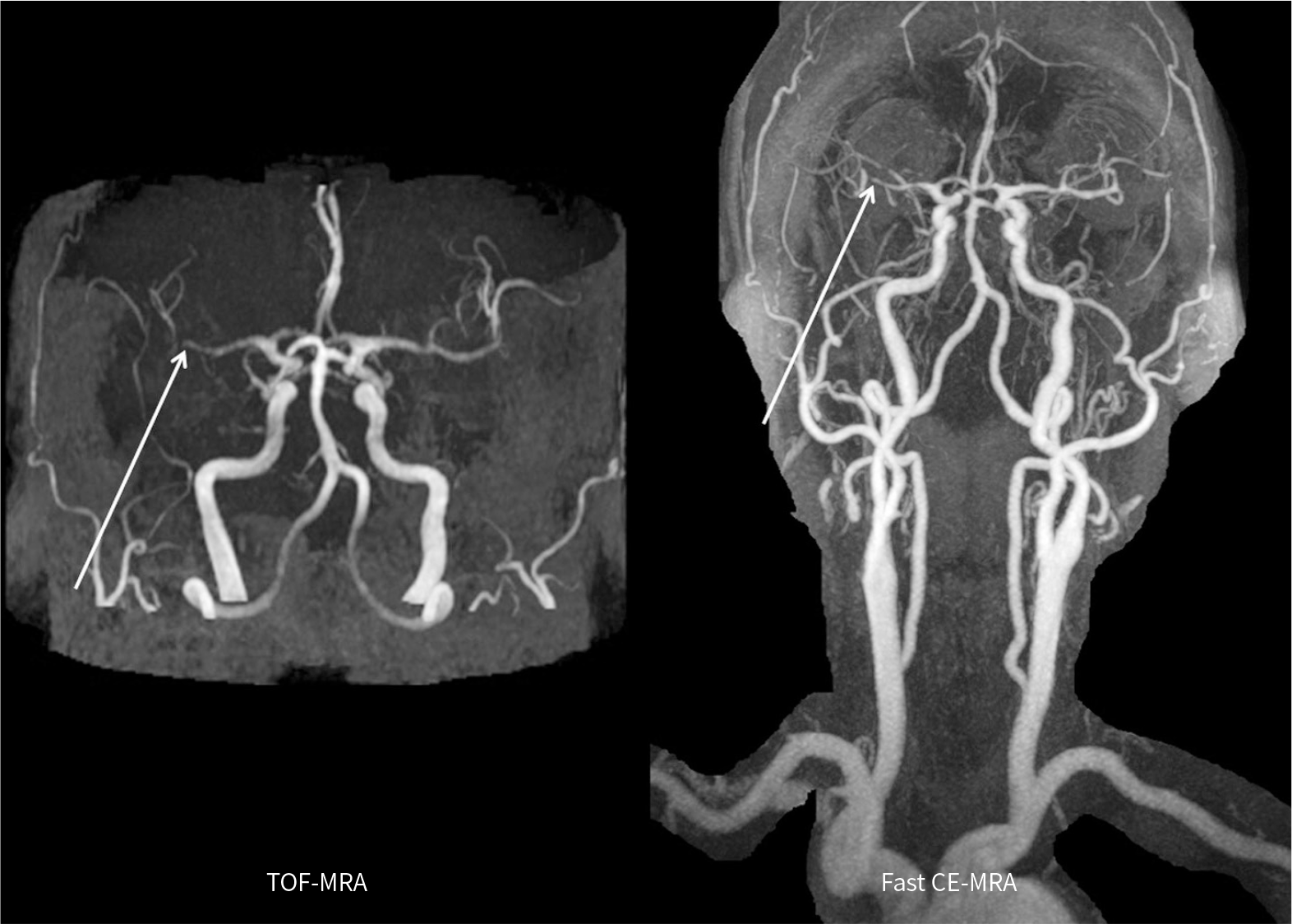




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download