Abstract
Purpose
Methods
Results
Conclusion
Figures and Tables
Fig. 1
Distribution of ABCD, DiaRem, and individualized metabolic surgery (IMS) scores in all participants. The total score of each scoring system is calculated by adding the points for each of the 4 variables (left panel). The cutoff values for each variable are shown. A, B, and C in the right panel indicate distribution of ABCD, DiaRem, and IMS scores in all participants, respectively. Intervals are 1 in ABCD score (A), 1 in DiaRem score (B), and 10 in IMS score (C). Subgroups with highly expected diabetes remission (>67%) are marked in panels A and B. BMI, body mass index; T2D, type 2 diabetes; SG, sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass.
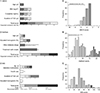
Fig. 2
Distribution of DiaRem (A) and ABCD (B) scores among patients in whom diabetes remission was highly expected according to the other system scores calculated by the other scoring system 52 and 82 patients were selected based on the ABCD and DiaRem scores, respectively. Patients with high expected remission rate with ABCD score show wide distribution of DiaRem score and vice versa, implying inconsistency between 2 scoring systems.
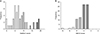
Table 1
Patients characteristics

Values are presented as mean ± standard deviation or number (%).
The mean homeostatic model assessment of insulin resistance represents the product of glucose and insulin concentrations divided by a factor.
BMI, body mass index; HOMA-IR, homeostasis model assessment-insulin resistance; IMS, individualized metabolic surgery.
a,b)Expected diabetes remission rates are 39.8% and 67.7%, respectively. c,d)Expected diabetes remission rate is 43.5%. e,f)Expected diabetes remission rate is 60%–70% after Roux-en-Y gastric bypass and 25%–56% after sleeve gastrectomy.
Table 3
Comparison of categories in which diabetes remission is highly expected

Values are presented as mean ± standard deviation or number (%).
BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; SE, standard error; NA, not applicable.
a,b)Expected diabetes remission rates are 43.5% and 39.8%, respectively [17]. c,d)Expected diabetes remission rates are 85.3% and 67.7%, respectively. e)Measure of agreement: <0 (poor), 0.0–0.2 (slight), 0.21–0.40 (fair), 0.41–0.60 (moderate), 0.61–0.80 (substantial), and 0.81–1.0 (almost perfect).
Table 4
Application of the individualized metabolic surgery (IMS) score system

Values are presented as mean ± standard deviation or number (%).
BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance.
The P-value was calculated by 1-way analysis of variance.
Mild stage: RYGB is recommended, and the expected remission rate after RYGB is 92%. Severe stage: Expected remission rates after both RYGB and SG are less than 15%. SG is recommended with expected remission rate of 12%. Moderate stage: RYGB is recommended, and the expected remission rate is 60%. a,b,c)The mean difference is significant at the 0.05 level.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download