Abstract
Objective
Methods
Results
References
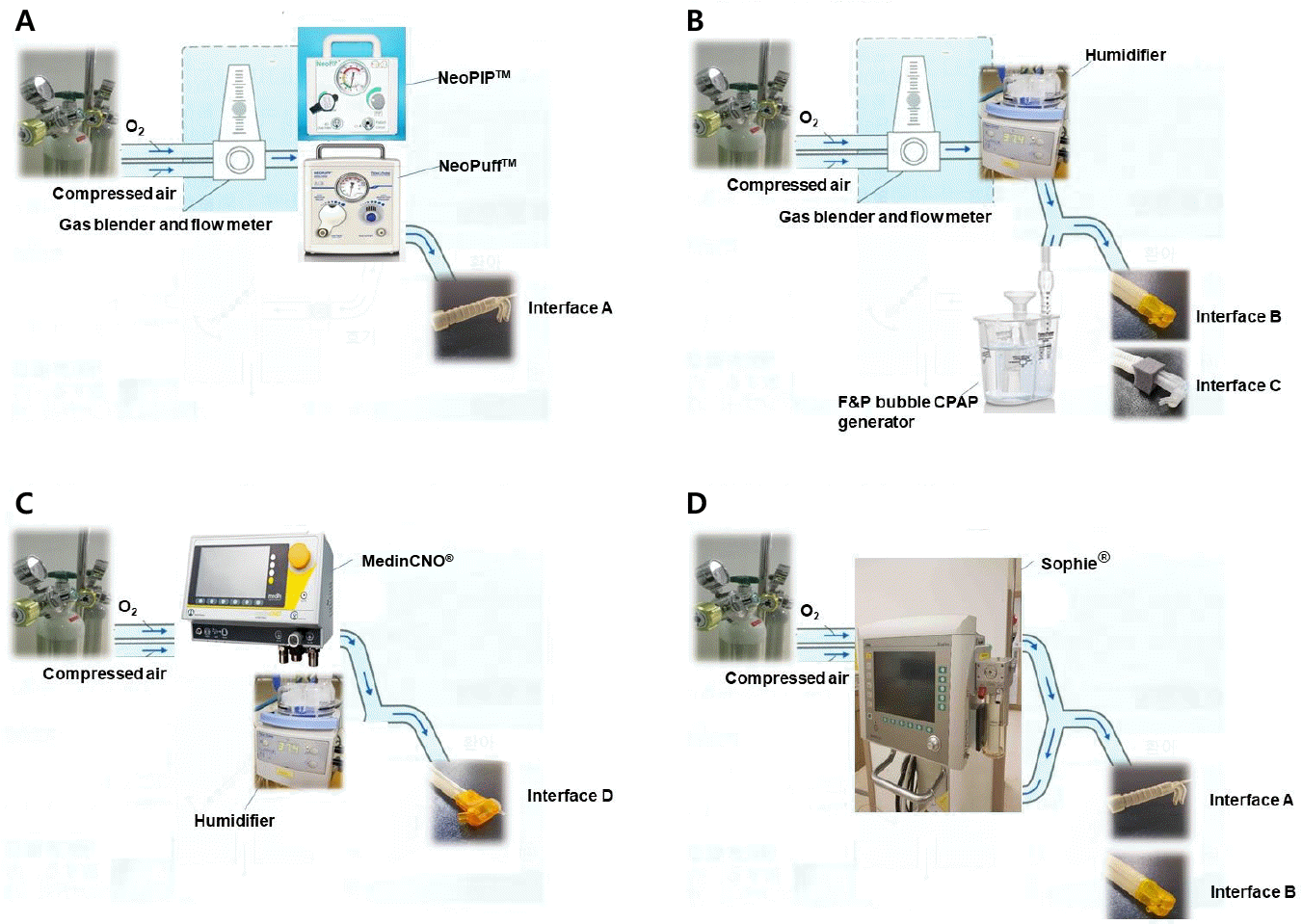 | Fig. 1.Schematic representation of the experimental equipment and circuits/interface(s). (A) Two T-piece resuscitators (NeoPuff TM [Fisher & Paykel Healthcare Ltd., Auckland, New Zealand] and NeoPIPTM [NeoForce Group Inc., Redwood City, CA, USA]) were connected to interface A via the non-humidified circuits. (B) The bubble CPAP (F&P bubble CPAP system [Fisher & Paykel Healthcare Ltd.]) was connected to interface B and C via a humidifier. (C) The flow-driven positive pressure equipment (medinCNO® [Medin Medical Innovations Gmbh, Munchen, Deutschland]) was connected to interface D. (D) The mechanical ventilator (Sophie® [Fritz Stephan Gmbh Medizintechnik, Gackenbach, Deutschland]) was connected to interface A and B. Interface A, Easyflow nCPAP® nasal cannula (Fritz Stephan Gmbh Medizintechnik); Interface B, Miniflow® generator and prong (Medin Medical Innovations Gmbh); Interface C, FlexiTrunkTM midline interface and nasal cannula (Fisher & Paykel Healthcare Ltd.); Interface D, Medijet® generator and prongs (Medin Medical Innovations Gmbh); CPAP, continuous positive airway pressure. |
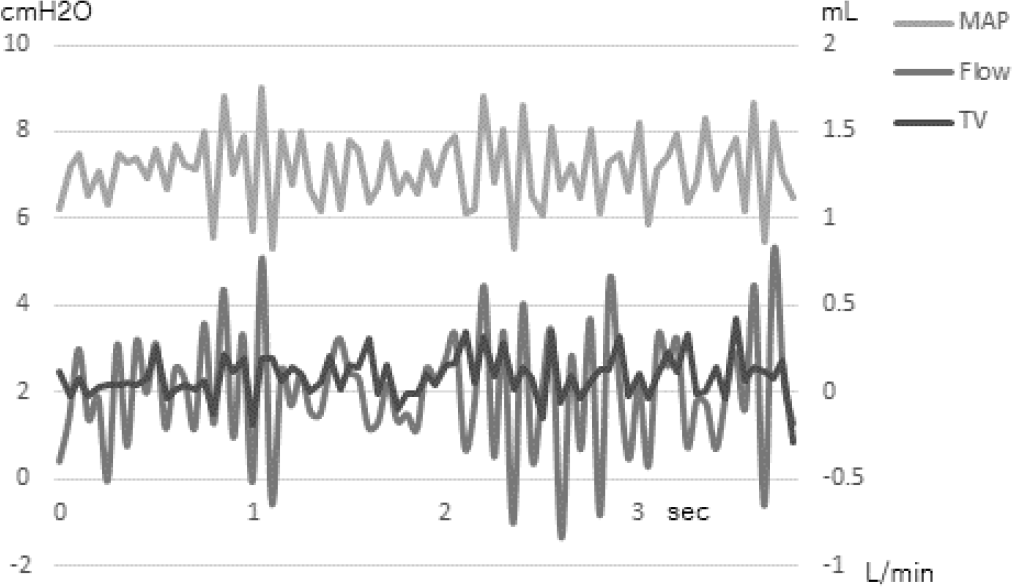 | Fig. 2.The waveforms of mean airway pressure (gray), airflow (dark gray) and delivered tidal volume (black) recorded by a lung simulator during non-breathing in the F&P bubble CPAP system (Fisher & Paykel Healthcare Ltd., Auckland, New Zealand). The pressure was set at 7 cm, and the flow was set at 7 liter/min. The pressure waveform of bCPAP showed intermittent increased pressure which was thought to be aroused by the water movement in the exhalation limb of the circuit. The waveform exhibited approximately 8 Hz frequency waves. MAP, mean airway pressure; TV, tidal volume; bCPAP, bubble continuous positive airway pressure. |
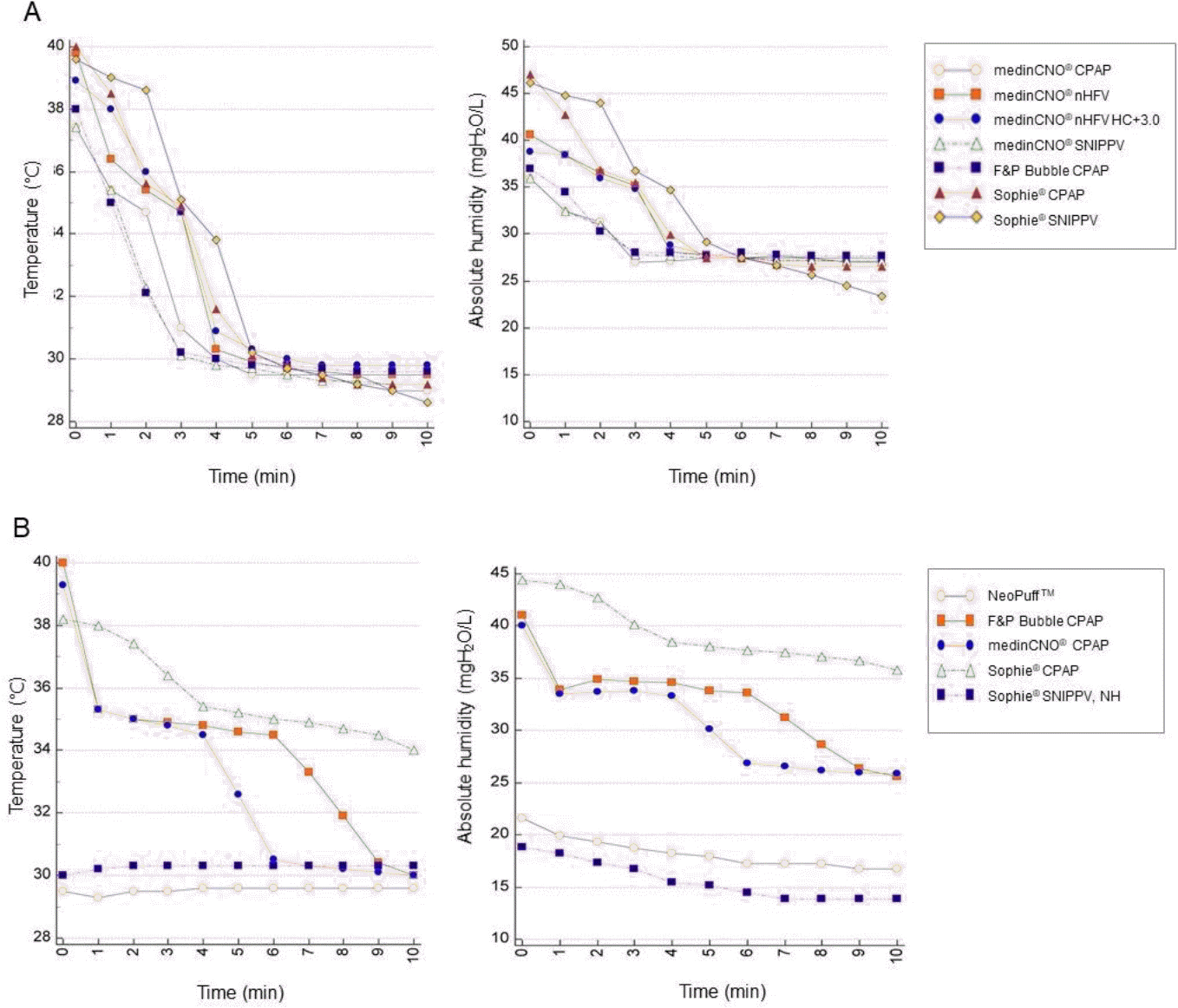 | Fig. 3.Time course of changes in temperature and absolute humidity. (A) On the open bassinet, the temperature dropped below 30°C, and the humidity dropped to about 25 mgH2 O/L in about 4 minutes in every mode of preheated equipment. (B) In the incubator, the temperature and absolute humidity were maintained for about 5 minutes and gradually dropped. In the NeoPuffTM (Fisher & Paykel Healthcare Ltd., Auckland, New Zealand) and non-heated/non-humidified Sophie® (Fritz Stephan Gmbh Medizintechnik, Gackenbach, Deutschland), the temperature remained around 30°C and the humidity also decreased, even though the proximal sensor was kept inside the incubator. CPAP, continuous positive airway pressure; nHFV, nasal high-frequency ventilation; HC, humidity compensation; SNIPPV, synchronized nasal intermittent positive pressure ventilation; NH, non-heated & non-humidified. |
Table 1.
Abbreviations: A, Easyflow nCPAP ® nasal cannula (Fritz Stephan Gmbh Medizintechnik, Gackenbach, Deutschland); B, Miniflow ® generator and prongs (Medin Medical Innovations Gmbh, Munchen, Deutschland); C, FlexiTrunkTM midline interface and nasal cannula (Fisher & Paykel Healthcare Ltd., Auckland, New Zealand); D, Medijet ® generator and prongs (Medin Medical Innovations Gmbh).
Table 2.
Abbreviations: SD, standard deviation; Pmean, mean pressure; A, Easyflow nCPAP® nasal cannula (Fritz Stephan Gmbh Medizintechnik, Gackenbach, Deutschland); B, Miniflow® generator and prongs (Medin Medical Innovations Gmbh, Munchen, Deutschland); D, Medijet® generator and prongs (Medin Medical Innovations Gmbh).*Fisher & Paykel Healthcare Ltd., Auckland, New Zealand.
Table 3.
Abbreviations: Pmax, maximum pressure; Pmin, minimum pressure; Pmean, mean pressure; SD, standard deviations; B, Miniflow ® generator and prongs (Medin Medical Innovations Gmbh, Munchen, Deutschland); C, FlexiTrunkTM midline interface and nasal cannula (Fisher & Paykel Healthcare Ltd., Auckland, New Zealand); D, Medijet® generator and prongs (Medin Medical Innovations Gmbh); A, Easyflow nCPAP® nasal cannula (Fritz Stephan Gmbh Medizintechnik, Gackenbach, Deutschland).
Table 4.
| Equipment | Interface | O2 flow (L/min) | Pressure (cmH2 O) | Simulated self-respiration | Measured Pmax (cmH2 O) | Measured Pmin (cmH2 O) | Measured Pmean±SD (cmH2O) | Delivered volume±SD (mL) |
|---|---|---|---|---|---|---|---|---|
| medinCNO ®* | D | 7+4 | No | 11.6 | 5.02 | 6.43±1.88 | 1.74±0.75 | |
| Yes | 11.8 | 5.14 | 6.83±2.10 | 2.28±0.42 | ||||
| Sophie®† | A | 15+5 | No | 14.6 | 4.49 | 7.75±4.07 | 4.28±0.05 | |
| Yes | 15.2 | 4.46 | 8.69±4.25 | 4.43±0.37 | ||||
| B | 15+5 | No | 14.6 | 4.51 | 7.57±3.68 | 4.11±0.07 | ||
| Yes | 16.1 | 4.59 | 8.28±3.88 | 4.33±0.34 |
Abbreviations: Pmax, maximum pressure; Pmin, minimum pressure; Pmean, mean pressure; SD, standard deviations; D, Medijet® generator and prongs (Medin Medical Innovations Gmbh, Munchen, Deutschland); A, Easyflow nCPAP® nasal cannula (Fritz Stephan Gmbh Medizintechnik, Gackenbach, Deutschland); B, Miniflow® generator and prongs (Medin Medical Innovations Gmbh).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download