Abstract
Placenta adhesion, often involving placenta accreta, placenta increta, and placenta percreta, is a clinical term used to describe placenta that does not separate spontaneously and cannot be removed without causing abnormally high blood loss. Prior cesarean section, other uterine surgery, assisted reproduction techniques and placenta previa are all risk factors for placental adhesion and their prevalence has increased steadily. Maternal mortality and morbidity are reduced when accurate prenatal diagnosis of placental adhesion is made. Currently, grayscale ultrasonography, with or without color Doppler has been used widely. However, the performance of these markers shows considerable variability and most of findings are poorly defined. The standardized ultrasonography description should always be reported when performing an ultrasonography scan for suspected placental adhesion to advance diagnosis and treatment. When suspicious findings are identified preoperatively, management should be tailored accordingly. The location and timing of delivery, access to a multidisciplinary care team, availability of the appropriate surgical approach, and access adjunctive techniques are key issues. In conclusion, placental adhesion is a clinically relevant, difficult-to-manage problem with rising incidence worldwide. Tertiary care hospital with an experienced optimum management should be considered and the basis for appropriate risk assessment and delivery planning improves maternal outcome.
Placental adhesion is a clinical term used to describe a placenta that fail to separate spontaneously at delivery. It encompasses the histopathologically defined diagnoses of placenta accreta, placenta increta and placenta percreta, which are based on how deeply the placenta is attached to the myometrium.
Placenta accreta was first described in the 20th century. In 1937, Irving and Hertig1 defined placenta accreta as “the abnormal adherence, either in whole or in part, of the after birth to the underlying uterine wall”.
Epidemiologic studies have shown a direct association between the increased prevalence of cesarean delivery and the increased incidence of placental adhesion in subsequent pregnancies.23 It has been shown that placental adhesion is associated with surgical damage to the integrity of the uterine lining, such as uterine curettage, manual delivery of the placenta, postpartum endometritis, previous hysteroscopic surgery, endometrial resection, and uterine artery embolization.4 However in the absence of surgical history, placental adhesion can be present with uterine pathology such as bicornuate uterus, adenomyosis, submucosal fibroids, and myotonic dystrophy.5
Over recent decades, the incidence of placenta adhesion has increased from 1 in 25,000 women in the 1950s to 1:2,500 in the 1980s, paralleling the concomitant rise in cesarean section and the rate of placental adhesion forecasts as high as 1:533 (USA) and 1:588 (Canada) by 2020 have been suggested recently.678 Among women undergoing primary cesarean section for indications other than placenta previa, the incidence of placenta accreta is approximately 0.03%. A second or subsequent cesarean section without placenta previa is associated with an increase in the risk of accreta. But incidence remains less than 1%, although the risk rises with each subsequent cesarean section.9 In women undergoing cesarean section for indications that include placenta previa, the risk of placenta accreta increases according to the number of prior cesarean sections. For example, one prior cesarean section plus placenta previa is associated with an 11% risk of placenta accreta, two prior cesarean sections the risk is 40%, and with three prior cesarean sections there is a 61% risk of placenta accrete.1011 These results indicate that the presence of a uterine scar is significantly associated with this increased risk.
The endovascular extravillous trophoblasts (EVT) invade the uterine wall as far as the inner third of the uterine myometrium. This superficial layer of the uterine musculature contains basal arteries that branch into the spiral arteries that supply the capillary plexus surrounding the uterine glands of the non-pregnant endometrium. EVT can be observed both within and around the spiral arteries in the central area of the placenta. They gradually extend laterally, reaching the periphery of the placenta around mid-gestation. Abnormal placental attachments to the uterine wall include placenta accreta which is characterized by invasion of trophoblasts into the myometrium; placenta increta which is characterized by deep myometrial invasion of trophoblast villi; and placenta percreta in which trophoblast villi perforate the full thickness of the myometrium and the uterine serosa with possible involvement of adjacent organs.
Abnormal decidualization in the area of a uterine scar allows abnormally deep trophoblastic infiltration and abnormal vascularization results from the post-surgery scarring process with secondary localized hypoxia leading to defective decidualization and excessive trophoblastic invasion.1213 In such cases, villous tissue invades deeply into the myometrium. And the myometrial muscle fibers can show degenerative changes such as increased fibrous tissue deposits and inflammatory cells infiltration (Fig. 1). In placenta accreta, direct apposition of villi to myometrium in absence of decidua may result in the presence of syncytial giant cells at the materno-placental interface.
Pathological studies have reported that, many EVTs are hypertrophic and that their numbers may increase and potentially appear as a thickened band at the implantation site.14 The band is frequently observed in placenta accreta. A large number of regulatory molecules have been demonstrated to have functional roles in the normal decidualization process and in controlling trophoblast adhesion, invasion, and the directionality of penetration.12 The processes of normal trophoblast invasion and placentation require precise regulation of vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and soluble fms like tyrosine kinase 1 (sFlt-1) expression; moreover, and oxygen tension has a key role in regulating their expression. Hypoxia stimulates both EVT proliferation and VEGF messenger ribonucleic acid (RNA) expression, while normoxia has an inhibitory effect on those action.15
Several biological factors such as levels of creatine kinase or elevated levels of alpha-fetoprotein have been suggested to placental dysfunction, but these hypotheses have not been confirmed.16 An increase in cell-free fetal DNA in maternal blood occur the invasion of trophoblasts into uterine muscle.717 Sekizawa et al.18 suggested that the presence of thin dysfunctional decidua may explain the increase in placenta previa cases. In their study,a higher concentration of fetal DNA was observed in the placenta previa group than in the control group.1819 Molecular level investigation procedures using maternal blood samples are available and they are capable of detecting placental abnormalities.20 However, the benefits, costs, and cost effectiveness of using these procedures should be considered before developing a routine biologic markers screening procedure. Assessment of relevant biological markers should be prospectively evaluated in selected high-risk groups to determine whether these biologic markers assays can provide sufficient additional benefits over current modes of diagnosis of placental dysfunction.
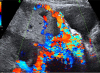
The primary screening method for identification of placenta accreta involves ultrasonography imaging. Women with a history of cesarean section or other uterine scarring should be carefully evaluated for sonographic features of placenta accreta. Maternal mortality and morbidity are reduced when placenta accreta is diagnosed antenatally and if the women subsequently deliver in a tertiary care hospital with a multidisciplinary care team.62122 There are commonly observed sonographic findings associated with placenta accreta. Gray-scale ultrasonographic features suggestive of placenta accreta include loss of a myometrial interface or retroplacental clear zone, chaotic intraplacental blood flow and intraplacental ‘lacunae’, and reduction of myometrial thickness to less than 1 mm.23 Moreover, the placenta is often visualized as bulging Perinatologytowards the bladder and in some cases placental invasion into the bladder can be observed. Numerous coherent vessels suggestive placenta accreta can be visualized by using 3-dimensional (3D) power Doppler sonography.910 This figure shows typical placenta lacunae which are the results of an anatomical disturbance of utero-placental circulation. Intraplacental sonolucent spaces, slow-moving maternal blood on gray-scale ultrasonography imaging form so called “placental lakes or moth-eaten” placental appearance (Fig. 2).4
The most commonly used diagnostic ultrasonographic signs are loss of a clear zone (98%) and the presence of placental lacunae (96.1%) on gray-scale image and the presence of sublacental hypervascularity (85.7%) and bridging vessels (61.9%) on color Doppler images.4 In addition, the loss of a hypoechogenic retroplacental zone, thinning or absence of the myometrial zone, thinning irregular or focal disruption of uteroplacental bladder zone and focal mass-like area of placenta echogenicity (exophytic) beyond the uterine serosa are meaningful signs of the depth of villous invasion in placenta accreta. Color Doppler imaging can improve the results of grayscale imaging of local vascular anatomy and can more clearly elucidate the normal and abnormal development of uterine circulation in early pregnancy. Doppler image features that are indicative of placenta accreta include chaotic intraplacental blood flow, the presence of altered blood flow in the retroplacental space and aberrant vessels crossing between placental surfaces.9122425
Magnetic resonance imaging (MRI) has also been used to evaluate placenta accreta. Some authors have suggested that MRI is better at defining the areas of abnormal placentation and assessing the depth of myometrial invasion than ultrasonographic image, particular in cases with posterior placentae. MRI scans can also help to diagnose obese women and assist in planning the surgical procedure at delivery.26 However, the use of MRI for screening as a routine clinical practice is limited due to its high cost compared to that of ultrasonography. Regardless, there are also limitations in ultrasonography. Much of diagnostic interpretation of ultrasonography images based on the subjective opinion of operator and is dependent on the operator's level of experience. Several studies have assessed the predictive value of different ultrasonography markers for diagnosing placenta accrete.21 The performance of these markers shows considerable variability, even though the studies are assessing the same markers.27 The sources of differences have ascribed to small sample size, retrospective study design and inconsistency in study inclusion criteria. To provide unified ultrasonography markers for placenta accreta to improve comparability of results of future studies and to increase diagnostic capabilities and to facilitate international collaboration, the European Working Group on Abnormally Invasive Placenta (EW-AIP) has proposed standardized definitions for AIP imaging descriptors.6 In addition, they proposed 3D power Doppler ultrasonography imaging for AIP assessment, and according to their recommendations, the standardizes set of ultrasonography signs should always be recorded and reported when performing an ultrasonography scan for suspected AIP.627
Using ultrasonography imaging in conjunction with the EW-AIP reporting method, our institute has reported on suspected placental adhesion. In addition, we are currently investigating correlations between parameters from ultrasonographic images of suspected AIP and various adverse clinical outcomes such as blood loss, transfusion status, and surgical approach. That study is underway and the data analysis and review are incomplete. However, there are some interesting early observations; for example, 58.3% of placenta lacunae and 83.3% of subplacental hypervascularity ultrasonography signs have occurred in concert with massive hemorrhage and the need for intensive medical care. These results indicate that the use a set of standardized ultrasonography descriptors in the diagnosis of placenta accreta is important because of the descriptor close association with clinical outcomes. However, the study is still underway and further our analysis is continuing.
Preoperative diagnosis of placenta accreta allows advanced surgical planning and preparation. Significant hemorrhage is common with placenta accreta, and it is likely that cesarean hysterectomy or uterine artery embolization will be required when placenta accreta is present. Therefore, women with suspected placenta accreta should be scheduled for delivery in a facility with appropriate surgical facilities and rapid access to a blood bank that can facilitate transfusion of large amounts of various blood products. These data are needed for management of peripartum hemorrhage in Korea from Health Insurance Review & Assessment Service. Among 1,600,000 births included in a big data review, the incidence of uterine artery embolization has increased while that of hysterectomy has decreased. In Korea in 2013, the incidence of uterine artery embolization was 1.13 per 1,000 births while the incidence of hysterectomy was 0.8 per 1,000 births. The most common risk factor for uterine artery embolization and peripartum hysterectomy in Korea is placenta previa, which is highly associated with placental adhesion. The most common risk factor for peripartum hysterectomy in Korea is placenta previa and it also has highly associated with the presence of placental adhesion.28
Key issues associated with placenta accreta are location and timing of delivery, access to a multidisciplinary care team, availability of the chosen surgical approach (hysterectomy or conservative management), and access adjunctive techniques. Eller et al.13 compared the multidisciplinary approach with standard obstetric care in women with placenta accreta and reported that early reoperation rate, transfusion volume, and overall morbidity were lower in the multidisciplinary group.13 Delivery at 34 weeks' gestation after antepartum steroids for fetal lung maturity is preferred but some cases without bleeding postpone delivery to 36 weeks of gestation.101329
A critical part of the transfer of care to a multidisciplinary team is the establishment of a point person or persons who will coordinate care and lead the formation and interaction of the multidisciplinary team. This responsibility is usually assumed by a maternal-fetal medicine specialist.10 Elective cesarean hysterectomy may be the safest approach, but patients with placenta accreta may desire conservative or uterine-preserving management; thus appropriate counseling should be provided early in the patient management process. Further the surgical team should include the most available and most experienced surgeons. We suggested that a combination of maternal-fetal medicine specialists and a gynecologic oncologist is optimal.9 Complex cases may require input from urologists or vascular and general surgeons. Ureteric stents have been advocated as a mean to avoiding ureteric injury in complex cases. Moreover, pelvic artery occlusion using balloon catheters has been advocated by several investigators.30 Prophylactic trans-catheter arterial balloon occlusion before delivery has been shown to reduced blood loss and transfusion requirement during surgery and postpartum management, and may prevent post-op uterine artery embolization, hysterectomy, and the requirement for intensive care unit admission.313233 While there is little overall effect on blood loss and catheter placement complications, including infection, thrombosis, and tissue necrosis, may be acceptable risks.133435 We continue to place catheters before surgery but the occlusive catheters are removed after surgery to reduce thrombotic complications. However one sheath is left in place overnight to facilitate targeted embolization should secondary hemorrhage occur.36
The principal reason for pursuing conservative treatment of placenta accreta is the preservation of fertility. In such cases, conservative treatment can includes stepwise uterine devascularization, intraoperative and postoperative embolization of pelvic vessels, methotrexate therapy, placental bed suturing, and intrauterine balloon occlusion. Failure of conservative treatment dose occurs and the rate of secondary hysterectomy is approximately 20%. Recent report on a series of 167 women with placenta accreta that were managed conservatively indicated that treatment was successful in 131 of the 167 cases.1537
In addition, as massive transfusion protocol should be in place so that additional blood products can be made available without delay in catastrophic hemorrhage cases. In massive hemorrhage, it is imperative that blood loss be actively managed and that early blood transfusion takes place before coagulopathy develops.
Recent advances in biology could allow prenatal screening of placenta accreta through the identification of biological markers in maternal blood obtained from cell-free fetal DNA, placental mRNA, and DNA microarray assay.16 These promising technologies can detect the presence of anomalies and should have a role in developing a better understanding of placental invasion.16
A potent antiangiogenic growth factor has been reported to be markedly elevated in preeclampsia and may also be associated with placental invasion.38 Moreover, it has been hypothesized that sFLT-1 regulates placental cytotrophoblast invasion and that low levels of sFLT-1 can occur locally in invasive placentas (accreta/increta/percreta). Shallow and excessive implantations have been associated with important pregnancy-related disease including preeclampsia and placental adhesion disorders. Interestingly, trophoblast invasion, which occurs during normal placental development, resembles the process of malignant cell invasion during tumorigenesis.39
Among the various microRNA (miRNA) species that are expressed in placental trophoblasts, members of the chromosome 19 miRNA cluster (C19MC) stand out due to their nearly exclusive expression in the placenta. Recent research on the function of C19MC miRNAs in normal cell physiology and during tumorigenesis identified one C19MC member, miR-519d, as a regulator of cell migration, cell invasion, and interaction with the extracellular matrix.40 In placental dysfunction disorders, such as preeclampsia and placental accreta, variety of miRNA species of the C19MC may be involved cell migration and invasion, as well as in interactions with extravillous trophoblasts. Further prospective studies that present standardized ultrasonography signs provide complete clinical and pathological data for individual cases, and undertake correlation analyses to determine relationship are essential. The identification of biological markers in maternal blood for use during prenatal screening for placenta accreta is essential for developing a more complete description of placental invasion.40
In conclusion, placenta accreta is a significant source of obstetric morbidity and mortality. Its incidence is increasing as a direct consequence of the increasing cesarean section rate. Optimum management for most placenta accreta cases requires elective cesarean hysterectomy, ideally performed at approximately 34 weeks of gestation. A multidisciplinary approach to placenta accreta treatment can produce the best outcomes.
Figures and Tables
References
1. Irving FC, Hertig AT. A study of placenta accreta. Surg Gynec Obst. 1937; 64:178.
2. Silver RM. Delivery after previous cesarean: long-term maternal outcomes. Semin Perinatol. 2010; 34:258–266.

3. Silver RM. Implications of the first cesarean: perinatal and future reproductive health and subsequent cesareans, placentation issues, uterine rupture risk, morbidity, and mortality. Semin Perinatol. 2012; 36:315–323.

4. Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016; 215:712–721.

5. Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997; 177:210–214.

6. Collins SL, Ashcroft A, Braun T, Calda P, Langhoff-Roos J, Morel O, et al. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016; 47:271–275.

8. Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005; 192:1458–1461.

9. Publications Committee, Society for Maternal-Fetal Medicine. Belfort MA. Placenta accreta. Am J Obstet Gynecol. 2010; 203:430–439.

10. Hull AD, Moore TR. Multiple repeat cesareans and the threat of placenta accreta: incidence, diagnosis, management. Clin Perinatol. 2011; 38:285–296.

11. Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006; 107:1226–1232.

12. Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012; 33:244–251.

13. Eller AG, Porter TF, Soisson P, Silver RM. Optimal management strategies for placenta accreta. BJOG. 2009; 116:648–654.

14. Greenberg JI, Suliman A, Iranpour P, Angle N. Prophylactic balloon occlusion of the internal iliac arteries to treat abnormal placentation: a cautionary case. Am J Obstet Gynecol. 2007; 197:470.e1–470.e4.

15. Timmermans S, van Hof AC, Duvekot JJ. Conservative management of abnormally invasive placentation. Obstet Gynecol Surv. 2007; 62:529–539.

16. Mazouni C, Gorincour G, Juhan V, Bretelle F. Placenta accreta: a review of current advances in prenatal diagnosis. Placenta. 2007; 28:599–603.

17. Zhou J, Li J, Yan P, Ye YH, Peng W, Wang S, et al. Maternal plasma levels of cell-free β-HCG mRNA as a prenatal diagnostic indicator of placenta accrete. Placenta. 2014; 35:691–695.

18. Sekizawa A, Jimbo M, Saito H, Iwasaki M, Sugito Y, Yukimoto Y, et al. Increased cell-free fetal DNA in plasma of two women with invasive placenta. Clin Chem. 2002; 48:353–354.

19. Beuker JM, Erwich JJ, Khong TY. Is endomyometrial injury during termination of pregnancy or curettage following miscarriage the precursor to placenta accreta? J Clin Pathol. 2005; 58:273–275.

20. Bartels HC, Postle JD, Downey P, Brennan DJ. Placenta accreta spectrum: a review of pathology, molecular biology, and biomarkers. Dis Markers. 2018; 2018:1507674.

21. Eller AG, Bennett MA, Sharshiner M, Masheter C, Soisson AP, Dodson M, et al. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet Gynecol. 2011; 117:331–337.

22. Chantraine F, Braun T, Gonser M, Henrich W, Tutschek B. Prenatal diagnosis of abnormally invasive placenta reduces maternal peripartum hemorrhage and morbidity. Acta Obstet Gynecol Scand. 2013; 92:439–444.

23. Comstock CH. Antenatal diagnosis of placenta accreta: a review. Ultrasound Obstet Gynecol. 2005; 26:89–96.

24. Wong HS, Cheung YK, Strand L, Carryer P, Parker S, Tait J, et al. Specific sonographic features of placenta accreta: tissue interface disruption on gray-scale imaging and evidence of vessels crossing interface-disruption sites on Doppler imaging. Ultrasound Obstet Gynecol. 2007; 29:239–240.
25. Dwyer BK, Belogolovkin V, Tran L, Rao A, Carroll I, Barth R, et al. Prenatal diagnosis of placenta accreta: sonography or magnetic resonance imaging? J Ultrasound Med. 2008; 27:1275–1281.
26. Nguyen D, Nguyen C, Yacobozzi M, Bsat F, Rakita D. Imaging of the placenta with pathologic correlation. Semin Ultrasound CT MR. 2012; 33:65–77.

27. D'Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013; 42:509–517.
28. Cho GJ, Kim LY, Hong HR, Lee CE, Hong SC, Oh MJ, et al. Trends in the rates of peripartum hysterectomy and uterine artery embolization. PLoS One. 2013; 8:e60512.

29. Robinson BK, Grobman WA. Effectiveness of timing strategies for delivery of individuals with placenta previa and accreta. Obstet Gynecol. 2010; 116:835–842.

30. Dubois J, Garel L, Grignon A, Lemay M, Leduc L. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood losses. Am J Obstet Gynecol. 1997; 176:723–726.

31. Hull AD, Resnik R. Placenta accreta and postpartum hemorrhage. Clin Obstet Gynecol. 2010; 53:228–236.

32. Bodner LJ, Nosher JL, Gribbin C, Siegel RL, Beale S, Scorza W. Balloon-assisted occlusion of the internal iliac arteries in patients with placenta accreta/percreta. Cardiovasc Intervent Radiol. 2006; 29:354–361.

33. Shih JC, Liu KL, Shyu MK. Temporary balloon occlusion of the common iliac artery: new approach to bleeding control during cesarean hysterectomy for placenta percreta. Am J Obstet Gynecol. 2005; 193:1756–1758.

34. Minas V, Gul N, Shaw E, Mwenenchanya S. Prophylactic balloon occlusion of the common iliac arteries for the management of suspected placenta accreta/percreta: conclusions from a short case series. Arch Gynecol Obstet. 2015; 291:461–465.

35. Chou MM, Kung HF, Hwang JI, Chen WC, Tseng JJ. Temporary prophylactic intravascular balloon occlusion of the common iliac arteries before cesarean hysterectomy for controlling operative blood loss in abnormal placentation. Taiwan J Obstet Gynecol. 2015; 54:493–498.

36. Cho YJ, Oh YT, Kim SY, Kim JY, Jung SY, Chon SJ, et al. The efficacy of pre-delivery prophylactic trans-catheter arterial balloon occlusion of bilateral internal iliac artery in patients with suspected placental adhesion. Obstet Gynecol Sci. 2017; 60:18–25.

37. Sentilhes L, Ambroselli C, Kayem G, Provansal M, Fernandez H, Perrotin F, et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010; 115:526–534.

38. Tseng JJ, Chou MM, Hsieh YT, Wen MC, Ho ES, Hsu SL. Differential expression of vascular endothelial growth factor, placenta growth factor and their receptors in placentae from pregnancies complicated by placenta accreta. Placenta. 2006; 27:70–78.

39. McMahon K, Karumanchi A, Stillman IE, Cummings P, Patton D, Eastering T. Dose soluble fms-like tyrosine kinase-1 regulate placental invasion? Insight from the invasive placenta. Am J Obstet Gynecol. 2014; 210:68.e1–68.e4.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download