Abstract
Perineal defect reconstruction is usually encountered in oncological conditions, trauma, or infection such as Fournier's gangrene. Reconstructive surgeons face challenges due to the complex structure and diversity of the different tissue and crucial organ components. In addition to innate characteristics, fecal contamination, difficulty of patient position to minimize trauma to the region, and suboptimal conditions for wound healing such as radiotherapy are other burdens to overcome. Common wound complications after perineal reconstruction are caused by remnant dead space, contamination, inadequate soft tissue volume, and chemoradiation-related entities after oncological resection. Classic methods such as direct closure or closure with local, regional, and distant pedicled flaps may often be unsuccessful. Free flap coverage has been reported to be successful, as the transferred tissue is outside the radiation field. We present a rare case of radiation-induced perineo-coccygeal defect covered with a free latissimus dorsi myocutaneous flap after abdominoperineal resection.
Extensive perineo-coccygeal defects can result from oncological resection of gynecological, urological, and colorectal tumors. The advancement of techniques in ablative surgeries has led to more-radical resections that leave larger defects1. At the same time, consideration for careful preservation of crucial structures that contribute various functions such as urination, defecation, sexuality, and reproduction cannot be neglected2. Defects that are irradiated for oncological management are especially more challenging because of the unfavorable conditions for wound healing and resistance to infection3. Placing a well vascularized muscle portion to fill a dead space can be helpful to prevent local infection4. Local flaps are often unavailable or complicated, which leaves surgeons with distant or free flaps as options1.
A 68-year-old male patient who was taking medications for diabetes and hypertension consulted the plastic and reconstructive surgery department because of a complicated perineal wound. It was a dehisced surgical site of a previously performed abdomino pelvic resection (APR), with the wound surface measuring 8×4 cm in size and a depth of 5 cm. Six months before, the patient was diagnosed as having stage IIA rectal cancer (carcinoembryonic antigen-positive adenocarcinoma with moderate vasculopathy). APR was performed 2 weeks before, after the patient underwent neoadjuvant concurrent chemoradiotherapy (CCRT) with Xeloda at a high dose of 50.4 Gy/28 Fx for 6 weeks.
The wound presented with purulent discharge and foul odor. The white blood cell (WBC) count was 22,000, and the C-reactive protein level was 17.3, which suggested a wound infection. Enterococcus faecalis and Enterococcus gallinarum were confirmed in the microbiology culture studies. Intravenous Ampicillin/Sulbactam was administered in accordance with the susceptibility studies and consultation with the department of infectious disease internal medicine. After initial debridement of the obviously infected and non-viable tissue, the WBC count and C-reactive protein level decreased to 11,000 and 1.7, respectively. Reconstructive measures were planned.
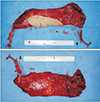
Under general anesthesia, the patient was placed in a prone position but slightly tilted toward the right side. The left portion of the upper extremity, the trunk, and both buttocks were draped and prepared. The preoperative photograph shown in Fig. 1 depicts the infectious slough covering the wound bed and deep dead space. Hydrosurgical debridement was performed using Versajet (Smith & Nephew, St. Petersburg, FL, USA), which was helpful to reach areas with restricted visualization. The intraoperative photograph presented in Fig. 2 shows the defect that had to be reconstructed. The final skin defect was 13×5 cm in size.
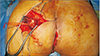
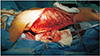
With a transverse incision across the left buttock, the superior gluteal artery and vein could be identified (Fig. 3). The pulsation and appearance of the vessels were evaluated, and we decided to use them as recipients for anastomosis. The left latissimus dorsi (LD) myocutaneous muscle was harvested in the same position (Fig. 4). The harvest flap included a skin paddle of 13×5 cm in size and muscle portion of 24×10 cm in size (Fig. 5). Thoracodorsal artery and vein were anastomosed end to end to the superior gluteal artery and vein. The muscle-portion of the flap was used for dead space obliteration, and the skin paddle was sutured to the defect without tension. The 6-month postoperative photographs in Fig. 6 show satisfactory results.
Reconstruction of the perineo-coccygeal region involves a complex anatomy, various internal organs, and a unique microbial environment. Defects are congenital or caused by trauma, infection, tumors, lymphedema, or other uncommon factors such as sex dysphoria. Regardless of the cause, restoration of the unique anatomy and function that can endure external pressure/friction is important. Even minor wound complications of reconstruction in the perineo-coccygeal region, unlike in other regions, can lead to poor hygiene and embarrassment, which can negatively impact self-esteem and quality of life2.
With the advancement of the multidisciplinary concepts of cancer of the perineal region that are aimed at a curative result, radical resection often leaves extensive defects5. Preoperative neoadjuvant CCRT has become the standard therapy for rectal cancer to downstage tumors6, followed by aggressive resection that may involve the pelvic viscera, blood vessels, muscles, or pelvic bone. Furthermore, maintaining quality of life over mere survival is the goal of modern oncological approaches. A successful reconstruction should recreate the three-dimensional anatomy, prevent functional loss, and sustain potential chemoradiotherapy1.
Previous radiotherapy and infection can complicate matters, like in the present case. Multiple factors such as an irradiated field (with neoadjuvant CCRT), remnant infection (due to the unique microbial environment, fecal contamination, and/or unfavorable vasculature of the irradiated tissue), deep dead space, and future chemoradiotherapies had to be considered. We presumed that local flaps would be suboptimal adjacent tissue. Furthermore, prolonged delay of reconstruction that can be beneficial for infected wounds can actually be detrimental, as it results in scar formation in the deep structures of the pelvic floor2.
Various forms of gluteal flaps such as rotation or advancement flaps can be used for relatively large defects. Perforator flaps based on the superior or inferior gluteal artery can also be used without significant donor-site morbidity. Pedicled muscle flaps can be useful for filling dead space while the well-vascularized muscle helps resist infection1. Successful reconstruction using the abovementioned flaps has been frequently reported. Nevertheless, some could argue that transferring tissue outside the radiation field is a better choice for patients who have received radiotherapy.
APR can be complicated with impaired wound healing, at an incidence of up to 26%. Distant metastasis, preoperative radiotherapy, tumor T-stage, smoking status, perioperative blood transfusion, preoperative chemotherapy, and side effects of preoperative chemoradiotherapies are risk factors of poor wound healing in APR. Hence, free flaps can be advantageous in these unfavorable settings7.
The LD free flap is well known for its easy dissection, reliability, large skin paddle, abundant muscle portion, long pedicle, large pedicle caliber, and versatility. Hence, it is advocated for the reconstruction of various defects such as those in the extremities and those created after head and neck cancers. The thin and pliable muscle portion can be used rather freely according to the defect, which can vary after debridement8. It can also be harvested with the patient in the prone position, which allows a two-team approach in cases of perineo-coccygeal defect reconstruction. This can be especially advantageous for patients who have undergone APR and CCRT, as their general conditions are likely to be suboptimal. Considering the remote location of the thoracodorsal artery and LD muscle from the radiation field, it may also be beneficial.
We believe the LD musculocutaneous flap can be a considerable option for patients with radiation-induced perineo-coccygeal defects after APR.
Figures and Tables
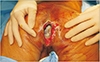 | Fig. 1Preoperative photograph of the perineo-coccygeal defect. Skin defect measured 8×4 cm. The dead space was 5 cm deep, covered with infectious slough and unhealthy granulation. |
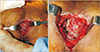 | Fig. 2Intraoperative photograph after debridement of non-viable tissue. The final skin defect was 13×5 cm. The dead space was approximately 6 cm deep. |
 | Fig. 3Intraoperative photograph of the recipient vessel. Left superior gluteal artery and veins were identified and confirmed to be patent. |
 | Fig. 4Intraoperative photograph of the donor site. Left latissimus dorsi musculocutaneous free flap was harvest in a prone position. The donor site was primarily closed. |
References
1. Brodbeck R, Horch RE, Arkudas A, Beier JP. Plastic and reconstructive surgery in the treatment of oncological perineal and genital defects. Front Oncol. 2015; 5:212.

2. Kolehmainen M, Suominen S, Tukiainen E. Pelvic, perineal and genital reconstructions. Scand J Surg. 2013; 102:25–31.

3. Artioukh DY, Smith RA, Gokul K. Risk factors for impaired healing of the perineal wound after abdominoperineal resection of rectum for carcinoma. Colorectal Dis. 2007; 9:362–367.

4. Anthony JP, Mathes SJ. The recalcitrant perineal wound after rectal extirpation. Applications of muscle flap closure. Arch Surg. 1990; 125:1371–1376. discussion 1376–7.
5. Winterton RI, Lambe GF, Ekwobi C, et al. Gluteal fold flaps for perineal reconstruction. J Plast Reconstr Aesthet Surg. 2013; 66:397–405.

6. Onaitis MW, Noone RB, Hartwig M, et al. Neoadjuvant chemoradiation for rectal cancer: analysis of clinical outcomes from a 13-year institutional experience. Ann Surg. 2001; 233:778–785.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download