Abstract
Purpose
The serologic diagnosis of rotaviral infections is not commonly used in clinical practice, but is used in seroepidemiologic studies. In this study, the usefulness of Escherichia coli-expressed recombinant VP6 proteins of group A rotavirus in the serodiagnosis of rotavirus infections by ELISA was evaluated.
Methods
The recombinant VP6 proteins of group A rotavirus expressed in E. coli Rosetta II strain were purified and identified. One hundred sera from 22 children (4 healthy neonates, 13 healthy children, and 5 immunocompromised children) who had serial sera samples prior to and after rotavirus infections were provided by the Gyeongsang National University Hospital, a member of the National Biobank of Korea. IgG, IgA, and IgM antibodies against rVP6 were analyzed by ELISA in all of the patients and Western blot analysis in 4 neonates.
Results
ELISA tests using rVP6 proteins of group A rotavirus as antigen revealed that IgG, IgA, and IgM antibodies increased after rotaviral infections in most neonates and healthy children. IgG antibodies also increased after rotaviral infections in most immunocompromised children without an adequate increase in IgM or IgA antibodies. Western blot analysis in four neonates revealed very early IgM antibody responses, even in the sera with low optical densities in ELISA tests.
Figures and Tables
 | Fig. 1The nucleotide sequences of rotavirus VP6 gene was obtained from the National Center for Biotechnology Information (NCBI) GenBank. |
 | Fig. 3The recombinant proteins from recombinant E. coli pET15b/rotavirus VP6 were analyzed by electrophoresis (12% SDS-PAGE). M: protein size marker, 1: E. coli pET15b/rotavirus VP6 whole cell lysate without IPTG induction, 2: E. coli pET15b/rotavirus VP6 whole cell lysate with IPTG induction, 3: supernatant of the whole cell lysate with IPTG induction, 4: precipitate of the whole cell lysate with IPTG induction. |
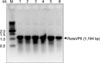
 | Fig. 4The purified refolded rotavirus VP6 proteins were analyzed by electrophoresis (12% SDS-PAGE). M: protein size marker, 1: flow through, 2: wash out, 3~8: fractions eluted with 20, 40, 80, 160, 320, and 500 mM imidazole, respectively. |
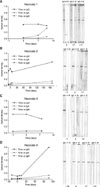 | Fig. 5Results of both ELISA and immunoblotting for detecting IgG, IgA, and IgM antibodies against rVP6 of rotavirus are shown in four healthy neonates who were diagnosed with rotaviral gastroenteritis. Sample date 0: the date when stool was confirmed positive for rotavirus antigen. |
 | Fig. 6Changes in optical densities of ELISA for detecting IgG, IgA, and IgM antibodies against rVP6 of rotavirus are shown in 13 healthy children who were diagnosed with rotaviral gastroenteritis. Sample date 0: the date when stool was confirmed positive for rotavirus antigen. |
 | Fig. 7Changes in optical densities of ELISA for detecting IgG, IgA, and IgM antibodies against rVP6 of rotavirus are shown in five immunocompromised children who were diagnosed with rotaviral gastroenteritis. Sample date 0: the date when stool was confirmed positive for rotavirus antigen. |
References
1. Chung JY. Acute viral gastroenteritis: recent trends and updates. Korean J Pediatr Gastroenterol Nutr. 2007. 10:Suppl 1. 53–57.
2. Kapikian AZ, Wyatt RG, Levine MM, Yolken RH, VanKirk DH, Dolin R, et al. Oral administration of human rotavirus to volunteers: induction of illness and correlates of resistance. J Infect Dis. 1983. 147:95–106.

3. Bishop RF, Barnes GL, Cipriani E, Lund JS. Clinical immunity after neonatal rotavirus infection. A prospective longitudinal study in young children. N Engl J Med. 1983. 309:72–76.

4. Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR, Glass RI. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993. 168:282–287.

5. Park SI, Kwon HO, Lee JH, Jung SJ. Clinical features of rotaviral gastroenteritis in neonates. Korean J Pediatr. 2005. 48:1121–1125.
6. Seo HJ, Jung YJ, Park SK, Choi SH, Lee JH, Kim MJ, et al. Rotavirus-associated neonatal necrotizing enterocolitis. Korean J Pediatr. 2009. 52:56–60.

7. Hwang PJ, Kwak JH, Lee TJ, Jeong SJ. Clinical features of acute noroviral gastroenteritis in children: comparison with rotaviral gastroenteritis. Korean J Pediatr. 2009. 52:453–457.

8. Iijima Y, Iwamoto T, Nukuzuma S, Ohishi H, Hayashi K, Kobayashi N. An outbreak of rotavirus infection among adults in an institution for rehabilitation: long-term residence in a closed community as a risk factor for rotavirus illness. Scand J Infect Dis. 2006. 38:490–496.

9. Kang KS, Shin KS, Cui XJ, Kim WY. VP7 genotypes of group A rotavirus isolated from infants and toddlers with rotavirus gastroenteritis in Jeju. Korean J Pediatr Gastroenterol Nutr. 2006. 9:147–152.

10. Ramani S, Arumugam R, Gopalarathinam N, Mohanty I, Mathew S, Gladstone BP, et al. Investigation of the environment and of mothers in transmission of rotavirus infections in the neonatal nursery. J Med Virol. 2008. 80:1099–1105.

11. Kim DS, Lee TJ, Kang JH, Kim JH, Lee JH, Ma SH, et al. Immunogenicity and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine in healthy infants in Korea. Pediatr Infect Dis J. 2008. 27:177–178.

12. Brüssow H, Werchau H, Liedtke W, Lerner L, Mietens C, Sidoti J, et al. Prevalence of antibodies to rotavirus in different age-groups of infants in Bochum, West Germany. J Infect Dis. 1988. 157:1014–1022.

13. Ward RL, McNeal MM. VP6: A candidate rotavirus vaccine. J Infect Dis. 2010. 202:Suppl. S101–S107.

14. Bishop RF, Cipriani E, Lund JS, Barnes GL, Hosking CS. Estimation of rotavirus immunoglobulin G antibodies in human serum samples by enzyme-linked immunosorbent assay: expression of results as units derived from a standard curve. J Clin Microbiol. 1984. 19:447–452.

15. Xu J, Dennehy P, Keyserling H, Westerman LE, Wang Y, Holman RC, et al. Serum antibody responses in children with rotavirus diarrhea can serve as proxy for protection. Clin Diagn Lab Immunol. 2005. 12:273–279.

16. Mukhopadhya I, Anbu D, Iturriza-Gomara M, Gray JJ, Brown DW, Kavanagh O, et al. Anti-VP6 IgG antibodies against group A and group C rotaviruses in South India. Epidemiol Infect. 2010. 138:442–447.

17. Hjelt K, Grauballe PC, Paerregaard A, Nielsen OH, Krasilnikoff PA. Protective effect of preexisting rotavirus-specific immunoglobulin A against naturally acquired rotavirus infection in children. J Med Virol. 1987. 21:39–47.

18. Velázquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000. 182:1602–1609.

19. Clemens JD, Ward RL, Rao MR, Sack DA, Knowlton DR, van Loon FP, et al. Seroepidemiologic evaluation of antibodies to rotavirus as correlates of the risk of clinically significant rotavirus diarrhea in rural Bangladesh. J Infect Dis. 1992. 165:161–165.

20. O'Ryan ML, Matson DO, Estes MK, Pickering LK. Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infections. J Infect Dis. 1994. 169:504–511.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download