Abstract
Backgrounds/Aims
Abdominal drains are routinely placed following Frey procedure for chronic pancreatitis (CP) despite the low incidence of pancreatic fistula (PF). The utility of the first postoperative day (POD1) drain fluid amylase (DFA) value in predicting PF in CP patients undergoing Frey procedure has not been previously reported.
Methods
A prospective study of patients with CP who underwent Frey procedure between August 2014 and April 2018. A standard technique of head coring with single layer continuous pancreatojejunostomy was done in all the patients. Amylase level of the drain placed close to the pancreatojejunostomy was recorded on POD1 and 3. Postoperative PF was defined and graded as per the updated International Study Group of Pancreatic Fistula (ISGPF) guidelines.
Results
Fifty-five patients with CP who fulfilled the inclusion criteria were included in the study. All had normal preoperative serum amylase level. Three patients developed a biochemical leak and four patients developed postoperative PF (Grade B - 3 and Grade C - 1). Receiver operating characteristics (ROC) curve identified a POD1 DFA cut-off value of 326 U/L that predicted a postoperative PF with sensitivity, specificity and negative predictive value of 100%, 70%, and 100% respectively.
Pancreatic fistula (PF) is a major cause of morbidity and mortality after pancreatic surgeries. Parenchymal consistency has been found to be an important risk factor for postoperative PF, with soft and non-fibrotic glands being at higher risk for a leak after pancreatic surgeries.1 Postoperative monitoring of drain fluid amylase (DFA) levels to detect the PF has been a routine practice among pancreatic surgeons. A few studies have shown that estimation of the first postoperative day (POD1) DFA can identify patients at low risk of PF following pancreatoduodenectomy and facilitate early drain removal.23 Frey procedure for chronic pancreatitis (CP) has increasingly become more popular as it combines resection (partial resection of the head of the pancreas) with drainage (lateral pancreatojejunostomy).4 Studies have shown that both pancreatoduodenectomy and Frey procedure provide good and permanent pain relief in patients with CP.56 However, pancreatoduodenectomy is associated with more perioperative morbidity and long-term mortality compared to Frey procedure.56 In patients with CP pancreas are generally firm to hard in consistency secondary to fibrosis, hence the incidence of PF is less compared to patients undergoing pancreatoduodenectomy.7 However, abdominal drains are routinely placed following Frey procedure for CP despite the low incidence of PF.47 Enhanced recovery after surgery (ERAS) with no drain protocol has been successfully employed in various gastrointestinal surgeries.8 However, pancreatic surgeons are reluctant to embrace the ERAS concept of no drain protocol in pancreatic surgeries including surgery for CP. The role of POD1 estimation of DFA in predicting the risk of PF in CP patients has not been previously reported. Hence, this prospective observational study was done to determine whether estimation of DFA levels on POD1 predicts PF in CP patients undergoing Frey procedure.
All patients with CP who underwent Frey procedure between August 2014 and April 2018 were included in this prospective cohort study. The study was approved by the Institute Scientific Advisory and Ethics committee. Patients with suspicion of malignancy and those who underwent distal pancreatosplenectomy along with the head coring were excluded from the analysis. Preoperative evaluation included an initial ultrasonography of the abdomen followed by cross-sectional imaging in the form of contrast-enhanced computed tomography or magnetic resonance imaging of the abdomen, or both. The indications for surgery in the present study were a history of pain attacks for at least one year requiring non-steroidal anti-inflammatory drugs (NSAIDS) or opioid analgesics, recurrent episodes of pain attacks (at least one per month requiring NSAIDS or opioid analgesics), or coexisting CP related complications like biliary stricture. A standard technique of head coring till the level of pancreatic duct to ensure adequate ductal decompression was performed in all patients. Pancreatojejunostomy was done using single layer continuous (using 3-0 polydioxanone) sutures in all the patients. As previously reported patients who had biliary obstruction secondary to CP underwent biliary drainage by Roux-en-Y hepaticojejunostomy.9 A single closed tube drain was placed near the pancreatojejunostomy site and brought out through left flank in all the patients. An additional drain was placed in the right subhepatic region for patients who underwent concomitant biliary drainage procedure.
Demographic parameters, body mass index (BMI), history of alcoholism and smoking, associated diabetes mellitus or steatorrhea, preoperative hematological and biochemical parameters, features in cross-sectional imaging, intraoperative findings including pancreatic duct diameter were recorded. Small duct disease was defined as pancreatic duct diameter less than 4 mm. In all patients, daily drainage output, drain fluid nature, and the levels of serum and drain fluid amylase were measured on post-operative day 1and 3. PF was defined and graded as per the updated International Study Group of Pancreatic Fistula (ISGPF) guidelines.10 Drains were removed by POD3 if DFA level was less than three times the upper limit of normal (laboratory normal upper limit of amylase level is 110 U/L). If the drains had not been removed, then measurements were made on post-operative day 5 and 7. As per the ISGPF definition, the patients who had normal DFA levels but had altered postoperative course associated with peripancreatic fluid collection requiring additional treatment including antibiotics, octreotide and invasive intervention were also included in the postoperative PF group. They were further categorized as Grade B or Grade C based on the type of intervention required. Postoperative hemorrhage was defined and graded as per International Study Group of Pancreatic Surgery (ISPGS) definition.11
The statistical tests were done using Statistical Package for the Social Sciences (SPSS) version 16. Non-parametric variables were compared using Fisher Exact test. A p-value of less than 0.05 was considered statistically significant. A ROC curve was constructed based on the sensitivity and specificity of POD1 DFA to detect postoperative PF.
Fifty-five patients who fulfilled the inclusion criteria and underwent Frey procedure during the study period were included in the analysis. Hepaticojejunostomy and choledochal cyst excision were performed in five and one patients with concomitant biliary stricture and choledochal cyst. The demographic and clinical parameters of CP patients included in the study is summarized in Table 1. The pain was the primary indication for surgery in all patients. The median (range) duration of pain was 15 (6–96) months. Twenty-one (38.2%) patients had pain episodes almost every day and the remaining patients had at least 2 episodes of pain in a month. Analgesics were used continuously by 32 (58.2%) patients and 18 (32.8%) patients were dependent on opioids. Thirty-eight (69.1%) patients had a history of hospitalization for pain attacks or episodes of exacerbation of pancreatitis, with a median (range) of 1.7 (1–11) hospitalizations per patient. Eleven (20%) patients received pancreatic enzyme supplement in the preoperative period. History of chronic alcohol intake was seen in 45% of patients; all of them were males, and the majority had consumed alcohol for more than ten years. Endocrine insufficiency (diabetes mellitus) was present in 44% of the patients, and 47% of patients were underweight (body mass index <18.5) for their age and height. None of the patients had a recent acute attack of pancreatitis (within four weeks) prior to surgery. Preoperative serum amylase level was within normal range in all patients.
The median operative time was 210 minutes, and median blood loss was 250 ml. There was no postoperative mortality. Three patients had biochemical leak and four patients developed postoperative PF that included Grade B in 3 patients and Grade C in one patient. The patient who had Grade C fistula required tracheostomy with prolonged mechanical ventilation and percutaneous drainage of the peripancreatic collection. Two out of the three patients who had Grade B fistula had Grade B post-pancreatectomy hemorrhage that was managed conservatively with blood transfusions. Of the seven patients with postoperative PF, only five patients had elevated DFA on POD3 (>330 U/L). In the other two patients (one Grade B and one Grade C) diagnosis of postoperative PF was based on clinical and radiological findings. The median (range) POD1 and POD3 DFA value of patients with a postoperative PF were significantly higher than in patients without a postoperative PF (Table 2). Considering the sensitivity and specificity of POD1 DFA value in detecting postoperative PF, an area under the ROC curve of 0.924 was obtained (p<0.001; 95% CI: 0.826–1.000) (Fig. 1). The POD1 DFA level of 326 U/L was identified as the best cut off to predict postoperative PF with the sensitivity and specificity of 100% and 70%. Of the 36 patients who had POD1 DFA <326 U/L, none had postoperative PF (Negative predictive value- 100%) (Table 3). On univariate analysis, only POD1 DFA>326 U/L was the significant predictor for pancreatic fistula after Frey procedure (Table 4).
Traditionally patients with CP undergo surgical intervention in a late stage of the disease when conservative medical treatment with analgesics and endoscopic interventions have failed. However, recent evidence suggests that surgical intervention early in the disease course results in better pain control with the potential of a reduced risk of pancreatic insufficiency and the need for further intervention.12 Studies and systemic reviews have shown that surgery offers better pain relief compared to endoscopic intervention or splanchnic nerve ablation.1314 Hence in the present series surgery was preferred as the primary treatment. Postoperative PF is the most frequent major complication occurring after pancreatic resections.1 The main reason behind routine drain placement after pancreatic resections is the fear that any unrecognised and untreated postoperative PF may lead to dreadful complications like the erosion of major blood vessels with haemorrhage, intra-abdominal abscess, sepsis, multisystem organ failure, and death.15 Recent studies have shown that intra-abdominal drains can increase the incidence of intra-abdominal and wound infections, exacerbate abdominal pain, reduce lung function, and prolonged hospitalization.16 Also, intra-abdominal drains were reported to erode the hollow viscera and peripancreatic vessels. Although some authors have suggested no drainage after standard pancreatic resections and few others support selective drain placement in high-risk patients, these views were not universally accepted.231718 In fact, a recent randomised controlled trial by Van Buren et al.19 have shown that routine elimination of intra-abdominal drainage after pancreatoduodenectomy increases the severity and frequency of complications and the risk of mortality. However early drain removal has been shown to significantly decrease morbidity including wound infection rate after pancreatic resection.20
Strong risk factors for postoperative PF after pancreatoduodenectomy (PD) include soft pancreas, small duct (MPD diameter <4 mm) and increased intraoperative blood loss.10 However, most of the studies on postoperative PF have been done in patients who underwent pancreatoduodenectomy or distal pancreatectomy.23 The incidence of postoperative PF after Frey procedure is generally low compared to pancreatoduodenectomy and distal pancreatectomy due to pancreatic fibrosis.47 In the present study, postoperative PF occurred in four out of 55 patients (7%) while three patients developed biochemical leak as per the updated ISGPF guidelines. The incidence of clinically relevant PF following Frey procedure for CP reported in various series is 5–7%.72122 Despite the low incidence of postoperative PF routine drain placement after Frey procedure is the rule among most of the surgeons. A reliable predictor of postoperative PF can facilitate early drain removal and avoid drain related morbidity in patients at low risk for postoperative PF.
Studies have shown that POD1 DFA can predict PF after pancreatic resections. Yamaguchi et al first reported that patients who developed PF had higher POD1 DFA levels.23 Molinari et al.2 in their series of 137 patients who underwent pancreatoduodenectomy (n=101) and distal pancreatectomy (n=36) reported that POD1 DFA>5000 IU/L indicated a higher risk of PF. Bassi et al.24 in their prospective randomized trial, showed that early removal of drains after standard pancreatic resections is advantageous in patients with POD1 DFA value <5000 U/L. However, a reliable POD1 DFA cut-off value to predict postoperative PF in patients undergoing Frey procedure has not been previously reported. As patients with CP have impaired glandular function secondary to chronic inflammation and pancreatic fibrosis DFA cut off value derived from patients undergoing pancreatoduodenectomy or distal pancreatectomy for malignant etiology may not be appropriate. In the present study, POD1 DFA cut-off value of 326 U/L predicted postoperative PF with a sensitivity and negative predictive value of 100%. Even the two patients who developed clinically relevant postoperative PF with normal DFA on the third postoperative day had elevated POD1 DFA.
ERAS pathways or “fast-track” protocols, which primarily aimed at sustainable improvements in patients care, both in terms of speed of recovery and quality were successfully employed in various gastrointestinal surgeries.8 Reports on the use of ERAS pathways in pancreatic surgeries are limited and are confined to retrospective and small prospective cohort studies.2526 The fear of PF precludes pancreatic surgeons from embracing the no drain protocol of ERAS pathway. Hence, modified ERAS protocol with early drain removal in patients with low POD1 DFA levels is suggested. Patients with CP who have a low risk of PF are the ideal group of patients where modified ERAS pathway can be successfully employed. The results of the present study suggest that the POD1 DFA cut off the value of 326 U/L can facilitate early drain removal in 65% (36/55) of patients undergoing Frey procedure for CP. A prospective study on the feasibility and safety of modified ERAS pathway with early drain removal utilizing the POD1 DFA value in patients with CP undergoing Frey procedure is currently underway in our Institute. The limitation of the present study is a relatively small sample size and a small number of patients with PF as the incidence of PF following surgery for CP is relatively less compared to surgery for periampullary tumors. Also, the use of updated ISGPF guidelines reduced the incidence of postoperative PF as biochemical leaks are no longer included in the definition of postoperative PF. The results of the present study would primarily help the surgeons who routinely place drain after Frey procedure.
In conclusion, the POD1 DFA is a reliable predictor of postoperative PF in CP patients who have undergone Frey procedure. The PF can be confidently excluded in patients who have a POD1 DFA less than 326 U/L, and such patients may be candidates for early drain removal.
Figures and Tables
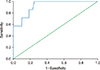 | Fig. 1Receiver operating characteristics (ROC) curve identified a POD1 DFA cut-off value of 326 U/l which predicted a PF with a sensitivity of 100% and specificity of 70%. Area under curve=0.924. |
Table 1
Demographic and clinical characteristics of patients with chronic pancreatitis included in the study

Table 2
Postoperative day (POD) 1 and 3 Drain fluid amylase (DFA) value in patients with and without pancreatic fistula

References
1. El Nakeeb A, Salah T, Sultan A, El Hemaly M, Askr W, Ezzat H, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg. 2013; 37:1405–1418.
2. Molinari E, Bassi C, Salvia R, Butturini G, Crippa S, Talamini G, et al. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg. 2007; 246:281–287.
3. Sutcliffe RP, Battula N, Haque A, Ali A, Srinivasan P, Atkinson SW, et al. Utility of drain fluid amylase measurement on the first postoperative day after pancreaticoduodenectomy. World J Surg. 2012; 36:879–883.
4. Roch A, Teyssedou J, Mutter D, Marescaux J, Pessaux P. Chronic pancreatitis: a surgical disease? Role of the Frey procedure. World J Gastrointest Surg. 2014; 6:129–135.
5. Izbicki JR, Bloechle C, Broering DC, Knoefel WT, Kuechler T, Broelsch CE. Extended drainage versus resection in surgery for chronic pancreatitis: a prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreatoduodenectomy. Ann Surg. 1998; 228:771–779.
6. Bachmann K, Tomkoetter L, Kutup A, Erbes J, Vashist Y, Mann O, et al. Is the Whipple procedure harmful for long-term outcome in treatment of chronic pancreatitis? 15-years follow-up comparing the outcome after pylorus-preserving pancreatoduodenectomy and Frey procedure in chronic pancreatitis. Ann Surg. 2013; 258:815–820. discussion 820–821.
7. Sudo T, Murakami Y, Uemura K, Hashimoto Y, Kondo N, Nakagawa N, et al. Short- and long-term results of lateral pancreaticojejunostomy for chronic pancreatitis: a retrospective Japanese single-center study. J Hepatobiliary Pancreat Sci. 2014; 21:426–432.
8. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017; 152:292–298.
9. Saluja SS, Kalayarasan R, Mishra PK, Srivastava S, Chandrasekar S, Godhi S. Chronic pancreatitis with benign biliary obstruction: management issues. World J Surg. 2014; 38:2455–2459.
10. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017; 161:584–591.
11. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007; 142:20–25.
12. Yang CJ, Bliss LA, Schapira EF, Freedman SD, Ng SC, Windsor JA, et al. Systematic review of early surgery for chronic pancreatitis: impact on pain, pancreatic function, and re-intervention. J Gastrointest Surg. 2014; 18:1863–1869.
13. Bhutiani N, Cheadle GA, Bahr MH, Vitale GC. Comparative efficacy of bilateral thoracoscopic splanchnicectomy for intractable pain secondary to pancreatic cancer vs chronic pancreatitis. J Am Coll Surg. 2017; 224:566–571.
14. Ahmed Ali U, Pahlplatz JM, Nealon WH, van Goor H, Gooszen HG, Boermeester MA. Endoscopic or surgical intervention for painful obstructive chronic pancreatitis. Cochrane Database Syst Rev. 2015; (3):CD007884.
15. Wang Q, Jiang YJ, Li J, Yang F, Di Y, Yao L, et al. Is routine drainage necessary after pancreaticoduodenectomy? World J Gastroenterol. 2014; 20:8110–8118.
16. van der Wilt AA, Coolsen MM, de Hingh IH, van der Wilt GJ, Groenewoud H, Dejong CH, et al. To drain or not to drain: a cumulative meta-analysis of the use of routine abdominal drains after pancreatic resection. HPB (Oxford). 2013; 15:337–344.
17. Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001; 234:487–493. discussion 493–494.
18. Fisher WE, Hodges SE, Silberfein EJ, Artinyan A, Ahern CH, Jo E, et al. Pancreatic resection without routine intraperitoneal drainage. HPB (Oxford). 2011; 13:503–510.
19. Van Buren G 2nd, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, et al. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014; 259:605–612.
20. Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006; 244:1–7.
21. Gestic MA, Callejas-Neto F, Chaim EA, Utrini MP, Cazzo E, Pareja JC. Surgical treatment of chronic pancreatitis using Frey's procedure: a Brazilian 16-year single-centre experience. HPB (Oxford). 2011; 13:263–271.
22. Tan CL, Zhang H, Yang M, Li SJ, Liu XB, Li KZ. Role of original and modified Frey's procedures in chronic pancreatitis. World J Gastroenterol. 2016; 22:10415–10423.
23. Yamaguchi M, Nakano H, Midorikawa T, Yoshizawa Y, Sanada Y, Kumada K. Prediction of pancreatic fistula by amylase levels of drainage fluid on the first day after pancreatectomy. Hepatogastroenterology. 2003; 50:1155–1158.
24. Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, et al. Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 2010; 252:207–214.
25. Berberat PO, Ingold H, Gulbinas A, Kleeff J, Müller MW, Gutt C, et al. Fast track--different implications in pancreatic surgery. J Gastrointest Surg. 2007; 11:880–887.
26. Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast-track recovery programme after pancreatico- duodenectomy reduces delayed gastric emptying. Br J Surg. 2008; 95:1387–1393.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download