Abstract
Backgrounds/Aims
Pancreatic leak and fistula formation following pancreatic resection is a dreaded complication associated with significant morbidity and mortality. The perioperative use of inotropes has been implicated in anastomotic dehiscence in other types of gastrointestinal surgery but their impact in pancreatic surgery remains unclear and a potentially modifiable risk factor for pancreatic leak. This study aims to assess the impact of perioperative inotrope infusion on the incidence of pancreatic leak following pancreaticoduodenectomy.
Methods
Retrospective data analysis of all patients undergoing pancreaticoduodenectomy at a tertiary HPB institute. Multivariate analysis and regression models assessed the impact of inotrope use against other known risk factors such as pancreatic duct size and gland texture. Pancreatic fistulae were graded as per ISGPF as Grade A (biochemical leak), Grade B and Grade C fistula.
Results
One-hundred and twenty-three (123) patients were included. A total of 52 patients (42%) developed a leak (29 grade A, 15 grade B, and 8 Grade C). In the fistula group, 28 patients (55%) received perioperative inotropes compared to 26 (35%) in the no fistula group. On univariate analysis, patients receiving inotropes (p=0.04) and patients with a soft pancreatic texture (p=0.003) had a statistically higher incidence of developing a pancreatic fistula of any grade. On multivariate analysis, only inotrope use was associated with an increased risk of developing a pancreatic fistula of any grade (OR 2.46, p=0.026), independent of pancreatic texture and pancreatic duct size.
The mortality following pancreatic resection has declined yet morbidity remains high.12 Postoperative pancreatic fistula is a significant and dreaded complication following pancreatic resection with a reported incidence of 5%–29%.3 Pancreatic fistula can result in sepsis, haemorrhage, prolonged hospital stay and death. Research focussing on modifiable risk factors remains of great interest to pancreatic surgeons striving to improve patient outcomes and identify high risk candidates. A soft pancreatic texture and small pancreatic duct have been consistently proven to be independent, albeit non-modifiable, risk factors for post-operative pancreatic leaks.345 Any factor which affects oxygen delivery to the peri-anastomotic microcirculation such as hypotension, anaemia, intraoperative blood transfusion and excessive crystalloid therapy have also been implicated in anastomotic dehiscence; primarily studied in colorectal surgery. With the widening use of Goal-Directed Fluid Therapy (GDFT) and Enhanced Recovery pathways (ERAS) for major gastrointestinal surgery the utilisation of perioperative inotropic support is common-place and is now written into anaesthetic ERAS algorithms for treating hypotension.6 Surgical concerns regarding the deleterious effect of inotropes on anastomotic healing are, however, based on a paucity of published evidence and continue to be debated. Theoretical vasopressor-induced splanchnic vasoconstriction can lead to shunting of microcirculation and local tissue hypoxia7 yet experimental animal models have failed to show any significant difference in anastomotic burst pressures or collagen tissue levels.8 More recently, clinical-based studies have shown that perioperative inotrope use may be associated with a 4-fold increase risk of developing a clinically significant anastomotic leak in a wide spectrum of gastrointestinal resection.910 This conflicting data means equipoise remains and further investigation is required. The aim of this study was to investigate whether perioperative inotrope infusion for major pancreatic surgery would independently impact on the incidence of pancreatic fistula formation.
This is a retrospective study which included patients undergoing pancreaticoduodenectomy (PD) between 2008 and 2017 in a single tertiary HPB institute. Patients undergoing total pancreatectomy, distal pancreatectomy and patients in whom data extraction could not be completed-were excluded. Retrospective review of the case notes and anaesthetic charts was conducted. Intraoperative surgeon-reported or pre-operatively radiologically reported duct size and parenchymal texture were recorded in addition to patient demographics, histopathology, estimated blood loss and need for blood transfusion. Perioperative inotrope use was defined as an infusion starting intraoperatively or within 24 hours of surgery and the duration of inotrope treatment was recorded. Diagnosis of post-operative pancreatic fistula was defined as per the updated International Study Groups (ISGPS) classification criteria of biochemical leak (Grade A), Grade B, or Grade C fistula.11 Pancrepancreatic surgeons from a single tertiary HPB referral centre. Pancreaticojejunostomy anastomosis was performed using either a dunking technique or a duct to mucosa anastomosis. All patients were routinely admitted to critical care post operatively and enrolled on ERAS pathway from 2014 onwards. Statistical analysis was performed using SPSS for Mac v20 (IBM Corp). Univariate analyses included chi-squared, unpaired t-tests, and Mann-Whitney U. Multivariate analyses were conducted using binary and ordinal logistic regression analyses. Statistical significance was set at p≤0.05.
A total of 123 patients were included in the study with a male to female ratio of 63:62. The median age was 67 (range 31–82) and median length of stay was 17 days (range 8–62). Indications for surgery were proven malignancy of the distal bile duct, pancreatic head or periampullary lesion in 89% of patients. Thirteen patients (11%) were found to have benign or premalignant lesions on final histopathological examination. Fifty-two patients (42%) developed a pancreatic leak. Twenty-nine of these were Grade A biochemical leaks and 23 were clinically significant (15 Grade B and 8 Grade C) leaks. The overall incidence of clinically significant fistulas following PD was 19%. In the fistula group, 28 patients (55%) received perioperative inotropes compared to 26 (35%) in the no fistula group.
The demographics of the two groups were similar. There was no significant difference between the median age, gender, haemoglobin, albumin, BMI, pancreatic duct size and texture or the median length of stay (Table 1).
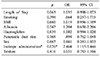
On univariate analysis, patients receiving inotropes (p=0.04) and patients with a soft pancreatic texture (p=0.003) had a statistically higher incidence of developing a pancreatic fistula of any grade. On multivariate analysis, only inotrope use was associated with an increased risk of developing a pancreatic fistula of any grade (OR 2.46, 95% CI 1.2–5.4, p=0.026), independent of pancreatic texture and pancreatic duct size (Table 2). For clinically significant fistula (Grade B and C) age, duct size, pancreatic texture and inotrope use was not associated with an increased risk of fistula formation. Interestingly, females were significantly less likely to develop clinically significant fistula compared to males (OR 0.169, 95% CI 0.05–0.58, p=0.005). Development of Grade B and C fistulas predictably resulted in a prolonged hospital stay (p=0.004) (Table 3).
The relationship between fistula formation and total inotrope duration (categorised as <24 hours, 24 to 48 hours, or >48 hours) was also investigated. Patients receiving inotropes for up to 24 hours were 2.5 times more likely to develop a leak of any grade compared to patients who did not receive any inotropes (OR 2.47, 95% CI 0.96–6.39, p=0.031). Patients receiving inotropes for 24–48 hours were over 4 times more likely to develop a leak of any grade (OR 4.18, 95% CI 0.28–13.7, p=0.018). This progressive relationship was not seen, however, in the small cohort of patients who received inotropes for over 48 hours post-operatively (n=4) (Table 4). On subgroup analysis of the 23 patients who developed Grade B and C clinically significant fistulas alone no relationship between inotrope duration and leak rate was demonstrated.
To our knowledge this is the first clinical study to demonstrate that utilisation of perioperative inotropes increases the risk of developing a pancreatic leak of any grade following pancreaticoduodenectomy. Furthermore, there appears to be a compound deleterious relationship with longer durations of use. The clinical relevance of this represents a potentially modifiable risk factor in pancreatic anastomotic healing.
A tension free and well perfused anastomosis are the cornerstones to a successful anastomosis. Experimental studies have shown that agents such as adrenaline and vasopressin significantly decrease superior mesenteric artery and microvascular flow due to vasoconstriction leading to an increase in gut lactate.71213 The translational clinical effects of this were further bridged by Sheridan et al. who showed reduced peri-anastomotic tissue oxygenation was predictive of anastomotic leak in 50 patients undergoing colonic resection.14
The impact of inotropes on developing clinically significant fistulas (only grade B and C) however, remains inconclusive as the low incidence of clinically significant fistulas in this study (23 fistulas) may lack the power to detect any statistically significant difference. This low incidence may also explain why no increase risk was detected in patients with a small caliber pancreatic duct; an independent risk factor shown in other studies.51516 Other weaknesses of this study include the bias inherently seen with retrospective data collection. In addition, two different anastomotic techniques were used by four different surgeons and was not factored into the analysis. However, previous studies have failed to show superiority of one technique over another.17
Our findings support that of a previous study of 223 patients admitted to the intensive care unit following any gastrointestinal (GI) surgery.10 They demonstrated a 3-fold increase in anastomotic leaks in all GI anastomoses when patients were treated with inotropes. The rates were higher when multiple agents were used for prolonged periods. The leak group, however, had a small sample size (just 26 patients). Furthermore, the leak group contained various types of GI anastomosis including ileocolic anastomoses. This cohort heterogeneity is likely to limit any conclusions drawn from this study but did call for evidence-based guidelines for postoperative use of inotropes. Another study of 140 major GI anastomotic leaks (40.9% of which were pancreatic resections) showed that as well as hypoalbuminaemia (<35 g/L), anaemia (<8 g/L), and need for blood transfusion, the use of intraoperative inotropes was independently associated with a four-fold increase risk of anastomotic leak (OR 4.11 95% CI 1.21–2.65, p=0.03).9 Although this retrospective study has the largest series of leaks to date, the group remains heterogeneous in terms of type of surgery and pathology. The type or duration of inotropes used was also not reported and pancreatic leaks were not analysed in isolation.
Improvements in mortality, length of hospital stay and complications seen with goal-directed fluid therapy and ERAS pathways means the utilisation of vasopressors to optimise perioperative haemodynamic parameters is likely to remain a key adjunct in pancreatic and GI surgery.1819 To date, the majority of published clinical data investigating the role of vasopressors and anastomotic complications tend to report deleterious effects. However, the validity of this evidence is compromised by small unpowered retrospective studies with inherent bias and confounding factors. Furthermore, studies which demonstrate no increased risk with inotropes may be under reported in the literature due to their negative findings. Only one recent study has reported the increasing use of vasopressors as part of an ERAS pathway for pancreatic surgery is not associated with a higher incidence of clinically significant pancreatic fistulas.6 This single-surgeon study of 132 patients (with 19 clinically significant fistulas) showed no difference in fistula rates between patients treated with inotropes and those who were not. Notably, their analysis included both pancreaticoduodenectomy and distal pancreatectomy; combining two operations with markedly different risk profiles for pancreatic leak. Furthermore, the study only assessed the intraoperative use of inotropes and, unlike the present data, did not assess the duration of treatment beyond the operating theatre. We believe the impact of inotropes on gastrointestinal healing goes beyond day 0 of surgery and should be considered.
Critics may suggest that vasopressor support is more commonly used in patients with less robust cardiovascular reserve and are simply a surrogate marker for high risk patients in general. In addition, withholding inotropes in lieu of an overall lower mean arterial pressure (MAP) may prove more deleterious for anastomosis and end-organ perfusion as a whole. The exact effect of inotropes in anastomotic leak after pancreaticoduodenectomy therefore remains inconclusive and drawing a direct causal link between the two should be done with caution. Perioperative variables and confounding factors together with small sample sizes means strong evidence to formulate clear guidelines in pancreatic surgery is not yet available. However, the evidence available to date including the current study would promote a more judicious use of vasopressors in the perioperative period.
Use of perioperative inotropes during pancreaticoduodenectomy is associated with an increased risk of pancreatic leak of all grades. This risk increases with longer durations of post-operative infusion (up to 48 hours). Larger studies are required before clear guidelines can be developed but current evidence would suggests inotropes should be used judiciously in the perioperative period after pancreatic resection.
Figures and Tables
Table 2
Multivariate binary logistic regression looking at variables affecting the odds of developing any grades of fistula (n=52)
References
1. Kagedan DJ, Ahmed M, Devitt KS, Wei AC. Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015; 17:11–16.
2. Amini N, Spolverato G, Kim Y, Pawlik TM. Trends in hospital volume and failure to rescue for pancreatic surgery. J Gastrointest Surg. 2015; 19:1581–1592.
3. Yeo CJ, Cameron JL, Lillemoe KD, Sauter PK, Coleman J, Sohn TA, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. 2000; 232:419–429.
4. Fuks D, Piessen G, Huet E, Tavernier M, Zerbib P, Michot F, et al. Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg. 2009; 197:702–709.
5. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013; 216:1–14.
6. Laks S, Isaak RS, Strassle PD, Hance L, Kolarczyk LM, Kim HJ. Increased intraoperative vasopressor use as part of an enhanced recovery after surgery pathway for pancreatectomy does not increase risk of pancreatic fistula. J Pancreat Cancer. 2018; 4:33–40.
7. Spronk PE, Zandstra DF, Ince C. Norepinephrine compromises intestinal microvascular perfusion? Intensive Care Med. 2004; 30:173–174. author reply 175.
8. Adanir T, Nazli O, Kara C, Aksun M, Sozutek A, Sencan A, et al. The relationship between vasopressor dose and anastomotic leak in colon surgery: an experimental trial. Int J Surg. 2010; 8:221–224.
9. Choudhuri AH, Uppal R, Kumar M. Influence of non-surgical risk factors on anastomotic leakage after major gastrointestinal surgery: audit from a tertiary care teaching institute. Int J Crit Illn Inj Sci. 2013; 3:246–249.
10. Zakrison T, Nascimento BA Jr, Tremblay LN, Kiss A, Rizoli SB. Perioperative vasopressors are associated with an increased risk of gastrointestinal anastomotic leakage. World J Surg. 2007; 31:1627–1634.
11. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017; 161:584–591.
12. Hiltebrand LB, Krejci V, Jakob SM, Takala J, Sigurdsson GH. Effects of vasopressin on microcirculatory blood flow in the gastrointestinal tract in anesthetized pigs in septic shock. Anesthesiology. 2007; 106:1156–1167.
13. Zhang W, Shibamoto T, Kuda Y, Shinomiya S, Kurata Y. The responses of the hepatic and splanchnic vascular beds to vasopressin in rats. Biomed Res. 2012; 33:83–88.
14. Sheridan WG, Lowndes RH, Young HL. Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum. 1987; 30:867–871.
15. Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007; 246:1058–1064.
16. Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010; 148:15–23.
17. Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA, et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003; 134:766–771.
18. Coolsen MM, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CH. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg. 2013; 37:1909–1918.
19. Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014; 38:1531–1541.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download