Abstract
REFERENCES
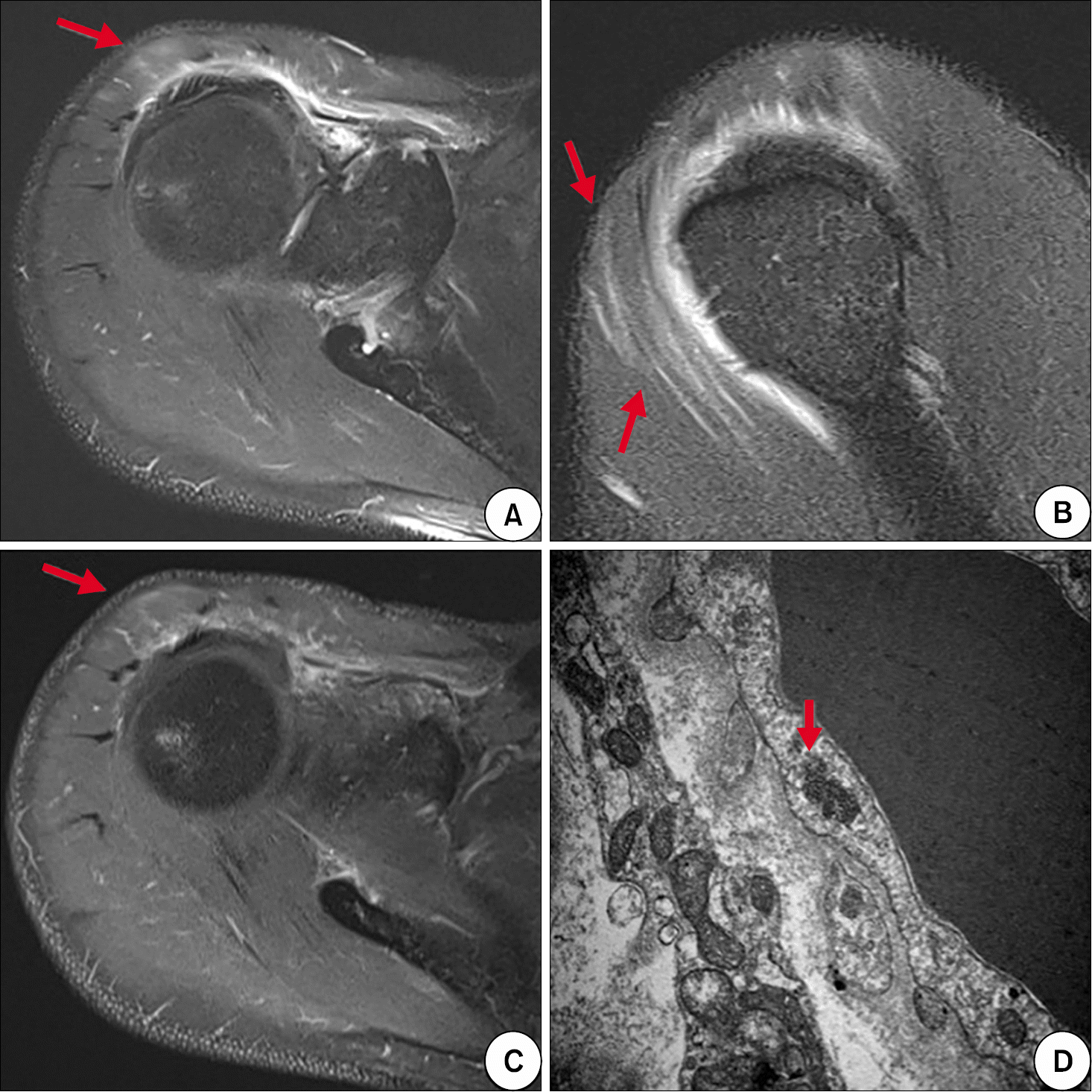 | Figure 1.T2-weighted magnetic resonance images (A, B) and enhanced T1-weighted magnetic resonance image (C) showed high signal intensity in right su-praspinatus and anterior deltoid muscles (arrows). (D) Electron microscopy of right deltoid muscle biopsy demonstrated focal infiltration of fat globules, focal areas of myofibrillar loss, and several tubuloreticular structures (arrow) in the cytoplasm of endothelial cells, which were consistent with dermatomyositis (×30,000). |
 | Figure 2.Initial chest computed tomography (CT) images showed multifocal patchy ground glass opacities (GGOs) in subpleural portion of both lower lungs (A, B). Follow-up chest CT images obtained 1 month after the initiation of therapy showed aggravation to reticulation with GGOs (C, D). Follow-up chest CT images obtained at the time of neuromyositis diagnosis showed improving state of previous noted reticulation and GGOs (E, F). |
Table 1.
| Variable | At the time of dermatomyositis diagnosis | At the time of neuromyositis diagnosis | After recovery of neuromyositis | Reference value |
|---|---|---|---|---|
| Clinical findings | ||||
| MMT grade (right/left) | ||||
| Shoulder abduction | 4-/4- | 5/5 | 5/5 | |
| Shoulder vertical flexion | 4-/4- | 5/5 | 5/5 | |
| Hip flexion | 4-/4- | 5/5 | 5/5 | |
| Ankle dorsiflexion | 5/5 | 5/5 | 3-/5 | |
| Ankle plantarflexion | 5/5 | 5/5 | 4-/5 | |
| Dyspnea (mMRC grade) | 0* | 0 | 0 | |
| Laboratory findings | ||||
| ESR (mm/hr) | 34 | 15 | 2 | 0∼10 |
| CRP (mg/dL) | 0.1 | 0.1 | 0.1 | 0∼0.5 |
| CK (U/L) | 344 | 136 | 129 | 56∼244 |
| AST (U/L) | 50 | 22 | 23 | 0∼37 |
| ALT (U/L) | 28 | 21 | 18 | 0∼41 |
| LDH (U/L) | 598 | 360 | - | 200∼400 |
| Pulmonary function test | ||||
| FVC, L (% predicted) | 4.76 (98) | 4.67 (96) | 5.03 (104) | |
| FEV1, L (% predicted) | 3.55 (95) | 3.56 (96) | 3.59 (97) | |
| FEV1/FVC, % | 75 | 76 | 71 | |
| DLco, mL/mmHg/min (% pred | dicted) 32.5 (129) | 37.1 (150) | 30.8 (122) |
MMT: manual muscle test, mMRC: modified Medical Research Council dyspnea scale, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, CK: creatine kinase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, FVC: forced vital capacity, FEV1: forced expiratory volume in 1 second, DLco: diffuse lung capacity for carbon monoxide.
Table 2.
Table 3.
The 2nd study means a study performed at the time of neuromyositis diagnosis. EHL: extensor hallucis longus, PL: peroneus longus, TA: tibialis anterior, Fib: fibrillation potentials, PSW: positive sharp waves, Fasc: fasciculation, Amp: amplitude, Dur: duration, Polyph: polyphasic, IP: interference pattern, PIP: partial interference pattern, CIP: complete interference pattern.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download