Abstract
Objective
Our aim was to investigate the long term safety and efficacy of etanercept in children with juvenile idiopathic arthritis (JIA).
Methods
The study subjects were the 90 JIA patients treated with etanercept in the Department of Pediatrics, Hallym University Medical Center between January 2004 and December 2017. We retrospectively reviewed their medical records for age at diagnosis, duration of etanercept treatment, number of active joints, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and adverse events during treatment.
Results
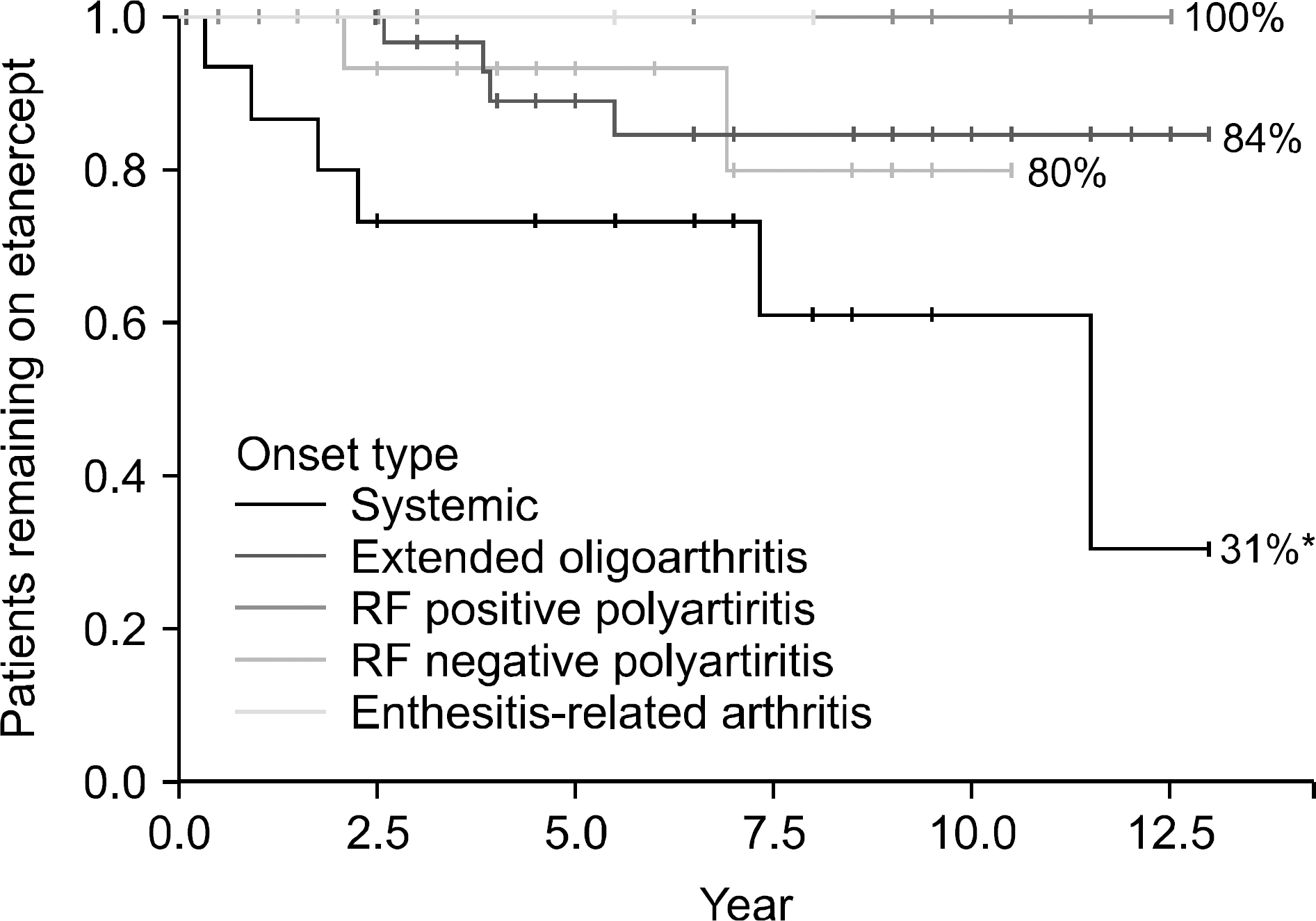
Among the 90 patients, 38 (42.0%) were male and 52 (58.0%) were female; 15 (16.7%) had systemic onset, 41 (45.6%) had extended oligoarticular, 14 (15.6%) had rheumatoid factor-positive polyarticular, 18 (20.0%) had rheumatoid factor-negative polyarticular, and 2 (2.1%) had enthesitis-related arthritis. The median age at the start of etanercept treatment was 9 years (range, 3∼18 years), and the median duration of etanercept treatment was 6 years (range, 0.5∼13 years). The median number of active joints decreased from 9 to 0 after 6 months of etanercept treatment. The median CRP and ESR were within normal range after 3 months of treatment. Six patients experienced recurrence, 9 switched to other medications and 3 discontinued etanercept. Of the 14 reported adverse events, 1 was serious, and there were no tuberculosis infections or malignancies.
Go to : 
REFERENCES
2. Peterson LS, Mason T, Nelson AM, O'Fallon WM, Gabriel SE. Juvenile rheumatoid arthritis in Rochester, Minnesota 1960-1993. Is the epidemiology changing? Arthritis Rheum. 1996; 39:1385–90.
3. Manners PJ. Epidemiology of the rheumatic diseases of childhood. Curr Rheumatol Rep. 2003; 5:453–7.

4. Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis why does it vary so much? J Rheumatol. 2002; 29:1520–30.
5. Cai Y, Liu X, Zhang W, Xu J, Cao L. Clinical trial of etanercept tapering in juvenile idiopathic arthritis during remission. Rheumatol Int. 2013; 33:2277–82.

6. Petty RE. Growing pains: the ILAR classification of juvenile idiopathic arthritis. J Rheumatol. 2001; 28:927–8.
7. Dueckers G, Guellac N, Arbogast M, Dannecker G, Foeldvari I, Frosch M, et al. Evidence and consensus based GKJR guidelines for the treatment of juvenile idiopathic arthritis. Clin Immunol. 2012; 142:176–93.

8. Kuemmerle-Deschner JB, Horneff G. Safety and efficacy of once-weekly application of Etanercept in children with juvenile idiopathic arthritis. Rheumatol Int. 2007; 28:153–6.

9. Suffredini AF, Reda D, Banks SM, Tropea M, Agosti JM, Miller R. Effects of recombinant dimeric TNF receptor on human inflammatory responses following intravenous endotoxin administration. J Immunol. 1995; 155:5038–45.
10. Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000; 342:763–9.
11. Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997; 337:141–7.

12. Moreland LW, Weinblatt ME, Keystone EC, Kremer JM, Martin RW, Schiff MH, et al. Etanercept treatment in adults with established rheumatoid arthritis: 7 years of clinical experience. J Rheumatol. 2006; 33:854–61.
13. Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008; 58:1496–504.

14. Southwood TR, Foster HE, Davidson JE, Hyrich KL, Cotter CB, Wedderburn LR, et al. Duration of etanercept treatment and reasons for discontinuation in a cohort of juvenile idiopathic arthritis patients. Rheumatology (Oxford). 2011; 50:189–95.

15. Favalli EG, Pontikaki I, Becciolini A, Biggioggero M, Ughi N, Romano M, et al. Real-life 10-year retention rate of first-line anti-TNF drugs for inflammatory arthritides in adult- and juvenile-onset populations: similarities and differences. Clin Rheumatol. 2017; 36:1747–55.

16. Neovius M, Arkema EV, Olsson H, Eriksson JK, Kristensen LE, Simard JF, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 2015; 74:354–60.

18. Woo P, Southwood TR, Prieur AM, Doré CJ, Grainger J, David J, et al. Randomized, placebo-controlled, crossover trial of low-dose oral methotrexate in children with extended oligoarticular or systemic arthritis. Arthritis Rheum. 2000; 43:1849–57.

19. Halle F, Prieur AM. Evaluation of methotrexate in the treatment of juvenile chronic arthritis according to the subtype. Clin Exp Rheumatol. 1991; 9:297–302.
20. Prince FH, Twilt M, ten Cate R, van Rossum MA, Armbrust W, Hoppenreijs EP, et al. Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis. 2009; 68:635–41.

21. Tzaribachev N, Kuemmerle-Deschner J, Eichner M, Horneff G. Safety and efficacy of etanercept in children with juvenile idiopathic arthritis below the age of 4 years. Rheumatol Int. 2008; 28:1031–4.
22. Bracaglia C, Buonuomo PS, Tozzi AE, Pardeo M, Nicolai R, Campana A, et al. Safety and efficacy of etanercept in a cohort of patients with juvenile idiopathic arthritis under 4 years of age. J Rheumatol. 2012; 39:1287–90.

23. Chiang YC, Kuo LN, Yen YH, Tang CH, Chen HY. Infection risk in patients with rheumatoid arthritis treated with etanercept or adalimumab. Comput Methods Programs Biomed. 2014; 116:319–27.

24. Ke WM, Chen LS, Parng IM, Chen WW, On AW. Risk of tuberculosis in rheumatoid arthritis patients on tumour necrosis factor-alpha inhibitor treatment in Taiwan. Int J Tuberc Lung Dis. 2013; 17:1590–5.

25. Lan JL, Chen YM, Hsieh TY, Chen YH, Hsieh CW, Chen DY, et al. Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann Rheum Dis. 2011; 70:1719–25.

26. Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, Lopez-Roses L, Brito-Zerón P, Pérez-de-Lis M, et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore). 2011; 90:359–71.
Go to : 
Table 1.
Clinical characteristics of patients treated by etanercept
Table 2.
Duration of etanercept treatment
Table 3.
Number of patients, active joints, CRP, and ESR levels at baseline and follow-up periods
Table 4.
Reasons for switching medication or discontinuing etanercept




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download