Dirofilaria immitis is a widespread filarial nematode found in temperate, subtropical, and tropical regions of the world [
1]. Mosquitoes are the most important vectors for infection with
D. immitis. Although its definitive hosts are primarily domestic and wild canids,
D. immitis shows low vertebrate host specificity, and is capable of infecting several mammalian species, including marine mammals [
2].
Japanese encephalitis virus (JEV), a mosquito-borne
Flavivirus in the family Flaviviridae, is a major cause of encephalitis in Southeast Asia and the Western Pacific region [
3]. Although the virus is transmitted via a zoonotic cycle between vector mosquitoes and pigs or birds as amplifiers, humans and horses can be incidentally infected, though they are considered dead-end hosts that cannot transmit the virus [
4].
Although several cases of D. immitis infection in captive pinnipeds have already been described, little is known about JEV infection in marine mammals. This report documents the first confirmed case of co-infection with D. immitis and JEV in a spotted seal (Phoca largha).
On August 18, 2017, a 10-year-old male spotted seal, suddenly showed diarrhea and anorexia. Of two seals reared in a wetland center in Jeollanam-do, the southern part of the Republic of Korea (ROK), one showed abnormal clinical signs. He was treated with antibiotics for a suspected intestinal disorders; however, his condition did not improve. After capturing the seal, he was treated with antibiotics in saline via IV injection, but eventually died 2 weeks later after experiencing convulsions. At necropsy, almost all the internal organs were severely congested. The lungs were incompletely collapsed and showed dark-red discoloration (
Fig. 1A). On the cut surface of the lungs, a large volume of frothy fluid oozed and several filarioid nematodes were observed in the vascular lumen (
Fig. 1B). The heart was enlarged and round, and adult filarioid nematodes were observed in the right ventricle (
Fig. 1C). The spleen was 3–5-fold larger than normal. No gross lesions were observed in the brain.
After necropsy, representative tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Then, 4-µm-thick sections were stained with hematoxylin and eosin for light microscopic examination. Immunohistochemistry (IHC) was also performed on brain sections to detect JEV antigens. Immunolabelling was performed using a rabbit polyclonal anti-JEV antibody (1:300; GeneTex, USA). Staining was performed using a fully automated system (NexES IHC instrument; Ventana Medical Systems, USA) and the DAB Detection System.
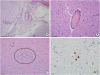
Histopathological examinations revealed moderate nonsuppurative meningoencephalitis (
Fig. 2A). The lesion severity was greatest in the cerebellum and brainstem, with less severe lesions identified in the cerebrum. In the leptomeninges and brain parenchyma, perivascular cuffs consisting of lymphocytes and macrophages were observed (
Fig. 2B). Multifocal glial nodules and occasional satellitosis and neuronophagia (
Fig. 2C) were identified in the cerebellar medulla and brainstem. The lungs showed moderate congestion, edema, and myointimal proliferation of the pulmonary arteries. However, no microfilaria were observed in the blood vessels of the internal organs. No histopathological findings were observed in any other organs. Through IHC, JEV antigen was detected primarily in the cytoplasm of necrotic neurons in the affected brain (
Fig. 2D).
Ten filarioid nematodes observed in the heart were carefully collected for parasitic species identification. Genomic DNA was extracted from homogenized worms using the QIAamp DNA Mini Kit (Qiagen, Germany). These filarioid nematodes were identified as
D. immitis using multiplex polymerase chain reaction (PCR) [
5].
Brain samples were examined for Eastern equine encephalitis virus (EEEV),
Flavivirus species including West Nile virus (WNV), and JEV using reverse transcription (RT)-PCR [
67]. Total RNA was extracted from the supernatant of 10% brain homogenate using a Maxwell instrument with a 16 LEV simple RNA purification kit (Promega, USA). RT-PCR was performed using the AccuPower RT-PCR PreMix & Master Mix kit (Bioneer, Korea). Brain samples were positive for
Flavivirus and negative for EEEV. The sequence of the identified
flavivirus had highest similarity to JEV based on NCBI-BLAST analysis.
For virus isolation, brain homogenates were inoculated with C6/36 cells derived from mosquito (ATCC CRL-1660; ATCC, USA). RNA was extracted from the supernatant of virus-infected C6/36 cells as mentioned above, and was subjected to RT-PCR with specific primers (
Supplementary Table 1). Sequencing was performed using an ABI system 3730xl DNA analyzer (Applied Biosystems, USA). The 5′- and 3′-terminal untranslated region (UTR) sequences of the viral genome were determined by rapid amplification of cDNA ends (RACE) (Clontech, USA). The full genome sequence of the isolate was compared with those of JEV strains from other countries in MEGA7 [
8] using a neighbor-joining method with 1,000 bootstrap iterations.
The full genome sequence of the JEV isolate, designated as JNSBr01/2017, was deposited in GenBank under accession number MK495877. The genome of the isolate was 10,964 nucleotides in length and consisted of an ORF with 10,299 nucleotides, encoding 3,431 amino acid residues. The 5′- and 3′-UTRs were 96 and 569 nucleotides, respectively. Based on phylogenetic analysis, JNSBr01/2017 strain was grouped with genotype I (GI) viruses from Korea, China, Japan, and Thailand (
Fig. 3). It showed high nucleotide (95.4%–99.5%) and deduced amino acid (87.3%–98.6%) sequence similarities with fully sequenced GI strains. The JNSBr01/2017 isolate was most closely related to the Chinese strain JS-1, with a maximum nucleotide sequence similarity of 99.5%.
Global warming and its impact on the spread of vector-borne diseases is a major concern in the ROK; although the country is located in a temperate zone [
1]. The ROK is a JE-endemic country that experiences seasonal outbreaks. The epidemic season usually begins in August, and the majority of cases are reported in the southern parts of the country [
9].
An attenuated live vaccine was developed and administered to both pigs and horses in the ROK in 1980 [
10]. Since then, the number of animal outbreaks has been significantly reduced. In the ROK, JE is a notifiable disease in swine only according to the Act on the Prevention of Contagious Animal Disease. There have been no official notifications of outbreaks in the Korean pig population since 2007. However, marine mammals are not vaccinated, and there have been no clinical reports of marine mammals infected with JEV until now.
The pathological lesions in the brain in this seal were similar to those described in horses and humans, in which nonsuppurative encephalitis with glial nodules and neuronophagia are common. A few viral, bacterial, and protozoal organisms can cause encephalomyelitis and/or meningoencephalitis in seals. Among these, virus-induced central nervous system diseases in pinnipeds are reports of infection with phocine distemper virus (PDV), canine distemper virus (CDV), WNV, and EEEV [
11]. Lesions associated with PDV and CDV infection include bronchointerstitial pneumonia and nonsuppurative demyelinating encephalitis with intranuclear and intracytoplasmic eosinophilic inclusion bodies in neurons and astrocytes [
11]. No pneumonia was observed in this seal, and the encephalitis lacked the typical morbilliviral inclusions. EEEV infection in seals is known to induce neutrophilic encephalitis, while WNV infectious cases are mononuclear in nature [
12]. These neurotropic viruses have been differentiated from JEV based on genetic findings.
Since 2010, a genotype shift of the circulating JEV strains in the ROK has occurred from G1 to GV [
13]. However, the isolate, JNSBr01/2017, was classified as G1 and had highest genetic similarity with the Chinese JEV strain (JS-1). JE is known to be endemic in Asian countries and is likely spread through the movement of infected birds or mosquitoes to new areas [
13]. Considering the JEV genotypes currently circulating in ROK and the genetic characteristics of the isolate, the possibility that the virus originated from other endemic regions, including China, cannot be excluded.
Neuro-virulence studies of JEV have demonstrated the E protein plays an important role in virulence during virus internalization. In particular, eight critical amino acids (E107, E138, E176, E177, E264, E279, E315, and E439) are closely linked to JEV virulence [
14]. A comparison of the critical amino acids in the E protein showed that the sequence of JNSBr01/2017 matched those of other virulent JEV strains (
Supplementary Table 2).
The seal was co-infected with
D. immitis worms. Most internal organs were congested and the lungs showed severe edematous changes. Although causative agents were identified as
D. immitis and JEV, it was unclear when these pathogens infected the seal. In the present case, several adult
D. immitis were observed in the heart and pulmonary vessels. When an infected mosquito bites a susceptible animal, third-stage
D. immitis larvae (L3) penetrate the host's skin and reach the blood vessels, where they develop until adulthood [
15]. It takes at least 6–8 months for a heartworm to invade the body and develop into an adult [
15]. However, JE typically develops in hosts after an incubation period of 5–15 days. The direct cause of death of this seal might have been circulatory disturbance due to
D. immitis infection. Moreover, JEV may have exacerbated the clinical signs in the seal.
Seals live in and/or close to water but they spend much time on land, where they can be bitten by mosquitos.
D. immitis is transmitted by culicid mosquito species such as
Anopheles sinensis,
Aedes vexans nipponii, and
Culex pipiens [
1], and JEV is primarily transmitted by the vector mosquito
Culex tritaeniorhynchus in the ROK [
13]. Although we could not confirm the cause of infection, it is speculated that the seal might have been infected with the two pathogens via separate mosquito bites.
D. immitis and JEV both show zoonotic potential [
23]. JEV is maintained in nature by mosquitoes, as well as wild birds and pigs, which act as important amplifiers of the virus. However, humans and horses do not transmit viruses back to mosquitoes, and are therefore considered dead-end hosts [
3]. Although the importance of seals in the epidemiology of JEV is unknown, its sporadic occurrence indicates that they, like horses, may be dead-end hosts. However, special precautions should be taken, as it is unclear whether the seal isolate has an altered host range or different pathogenic properties. Above all, it is necessary to reduce the populations of mosquitoes, the mediators of these diseases, to prevent their spreading.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download