Hepatitis B virus (HBV) infection is a major global health burden.
1 An estimated 291 million people are chronically infected with HBV, a leading cause of cancer-related mortality worldwide.
2
Statins, also known as 3-hydroxy-3-methylglutaryl CoA reductase inhibitors, are widely used for treating dyslipidemia and preventing cardiovascular disease.
3 Studies have reported a decreased risk of cancers after the use of statins, including breast cancer, colon cancer, and prostate cancer.
45 The chemopreventive effects of statins may be related to growth and apoptosis effects on cancer cells.
6 The beneficial effect of statin use on the development of hepatocellular carcinoma (HCC) in patients with chronic HBV infection, HCV infection, or cirrhosis has been reported.
78910 In addition, a previous study reported an association between statin use and a reduced risk of liver cancer and death among cirrhosis patients with a focus on hypercholesterolemia.
11 However, limited data are available on whether the same beneficial effects of statin use can be expected in patients with chronic hepatitis B (CHB) without cirrhosis when hypercholesterolemia or obesity are considered as confounding factors. Therefore, the present study sought to investigate the effect of statin use on liver cancer mortality in non-cirrhotic patients with CHB taking hypercholesterolemia and obesity into account.
A nationwide retrospective cohort study was conducted using data from a Health Examination Cohort of the National Health Insurance Service (NHIS) of Korea. The data collection method was the same as that in a previous study.
12 A representative sample cohort of 514866 individuals was randomly selected from 5.15 million Koreans aged 40–79 years who underwent health examinations between January 1, 2002 and December 31, 2003. Patients with CHB (n=22987) were selected from the database using the disease codes B18.0 or B18.1. Statin use was defined as a cumulative defined daily dose (cDDD) ≥28 between 2003 and 2005.
7 In order to avoid immortal time bias, we utilized the landmark analysis method. For patients who started statin therapy within this 3-year landmark date, the patients were designated into statin users. Both statin users and non-users were followed up from the landmark date to 2013. The primary outcome was liver cancer-related death and the disease code used was C22 (liver cancers). This study was approved by the Institutional Review Board of Kyung Hee University (KHSIRB-16-048).
The exclusion criteria were as follows: history of alcoholic liver disease or chronic hepatitis C (B 18.2) (n=5388); liver cirrhosis (K74), liver cancer (C22), or other chronic liver diseases (B18.8, B18.9) at baseline (n=3890); patients who had been treated with statins before the health examination (n=127); patients who were treated with statins cDDD ≥28 between January 2002 and December 2002 (n=303); patients on other dyslipidemic agents before the health examination (n=10); and patients on other dyslipidemic agents between January 2002 and December 2002 (n=37). Also, any patients who died during the statin exposure period were excluded (n=169). A total of 13063 patients with CHB were enrolled. The baseline characteristics and laboratory test results of the study population were collected from the date closest to the date of the CHB diagnosis within 2 years. In cases wherein no data were available, we collected data within 1 year after the CHB diagnosis.
The characteristics of statin users were compared with those of non-users. Categorical and continuous variables were compared using the chi-square and t-test, respectively. Cumulative rates of liver cancer mortality were estimated using the Kaplan-Meier method and log lank test. Univariate and multivariable Cox proportional hazards regression analysis were performed to assess hazard ratios (HRs) of liver cancer mortality. Age, sex, body mass index (BMI), serum alanine aminotransferase (ALT) levels, γ-glutamyl transferase (GGT) levels, total cholesterol levels, fasting blood glucose levels, hypertension, family history of liver disease, smoking status, alcohol consumption, and statin use were included in multivariable analyses. All analyses were performed using SAS version 9.4 (SAS Institute; Cary, NC, USA). Two-sided p values<0.05 were considered to indicate statistical significance.
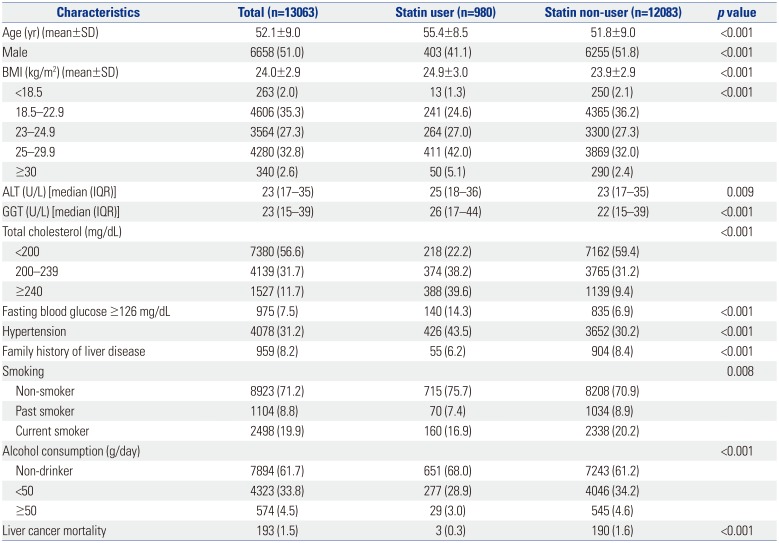
The baseline characteristics of the study population are shown in
Table 1. Of the 13063 patients, 980 (7.5%) were statin users and 12083 (92.5%) were statin-naïve. Statin users were older (mean age, 55 vs. 52 years,
p<0.001), had higher BMI (24.9 vs. 23.9,
p<0.001), had higher levels of ALT (25 vs. 23 U/L,
p=0.009) and GGT (26 vs. 22 U/L,
p<0.001), and had a higher proportion of hypertension (43.5% vs. 30.2%,
p<0.001), compared with non-users. The number of patients having total cholesterol levels <200 mg/dL, 200–239 mg/dL, and ≥240 mg/dL were 218 (22.2%), 374 (38.2%), and 388 (39.6%), respectively, among statin users and 7162 (59.4%), 3765 (31.2%), and 1139 (9.4%), respectively, among non-users.
Table 1
Baseline Characteristics of the Study Patients
|
Characteristics |
Total (n=13063) |
Statin user (n=980) |
Statin non-user (n=12083) |
p value |
|
Age (yr) (mean±SD) |
52.1±9.0 |
55.4±8.5 |
51.8±9.0 |
<0.001 |
|
Male |
6658 (51.0) |
403 (41.1) |
6255 (51.8) |
<0.001 |
|
BMI (kg/m2) (mean±SD) |
24.0±2.9 |
24.9±3.0 |
23.9±2.9 |
<0.001 |
|
<18.5 |
263 (2.0) |
13 (1.3) |
250 (2.1) |
<0.001 |
|
18.5–22.9 |
4606 (35.3) |
241 (24.6) |
4365 (36.2) |
|
|
23–24.9 |
3564 (27.3) |
264 (27.0) |
3300 (27.3) |
|
|
25–29.9 |
4280 (32.8) |
411 (42.0) |
3869 (32.0) |
|
|
≥30 |
340 (2.6) |
50 (5.1) |
290 (2.4) |
|
|
ALT (U/L) [median (IQR)] |
23 (17–35) |
25 (18–36) |
23 (17–35) |
0.009 |
|
GGT (U/L) [median (IQR)] |
23 (15–39) |
26 (17–44) |
22 (15–39) |
<0.001 |
|
Total cholesterol (mg/dL) |
|
|
|
<0.001 |
|
<200 |
7380 (56.6) |
218 (22.2) |
7162 (59.4) |
|
|
200–239 |
4139 (31.7) |
374 (38.2) |
3765 (31.2) |
|
|
≥240 |
1527 (11.7) |
388 (39.6) |
1139 (9.4) |
|
|
Fasting blood glucose ≥126 mg/dL |
975 (7.5) |
140 (14.3) |
835 (6.9) |
<0.001 |
|
Hypertension |
4078 (31.2) |
426 (43.5) |
3652 (30.2) |
<0.001 |
|
Family history of liver disease |
959 (8.2) |
55 (6.2) |
904 (8.4) |
<0.001 |
|
Smoking |
|
|
|
0.008 |
|
Non-smoker |
8923 (71.2) |
715 (75.7) |
8208 (70.9) |
|
|
Past smoker |
1104 (8.8) |
70 (7.4) |
1034 (8.9) |
|
|
Current smoker |
2498 (19.9) |
160 (16.9) |
2338 (20.2) |
|
|
Alcohol consumption (g/day) |
|
|
|
<0.001 |
|
Non-drinker |
7894 (61.7) |
651 (68.0) |
7243 (61.2) |
|
|
<50 |
4323 (33.8) |
277 (28.9) |
4046 (34.2) |
|
|
≥50 |
574 (4.5) |
29 (3.0) |
545 (4.6) |
|
|
Liver cancer mortality |
193 (1.5) |
3 (0.3) |
190 (1.6) |
<0.001 |

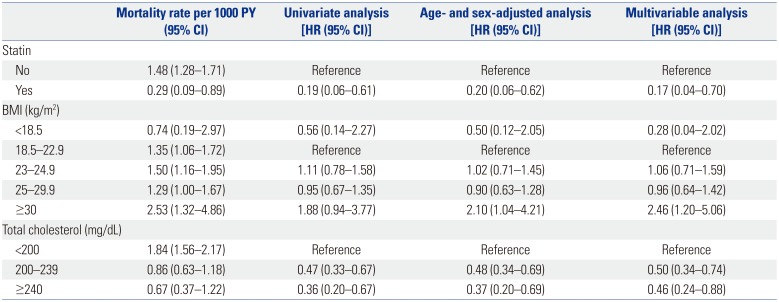
During the mean follow-up period of 10.6 years (standard deviation, 1.2 years), 193 patients (1.5%) died of liver cancer. The annual liver cancer mortality was significantly lower among statin users [0.29 per 1000 person-year (PY)] than non-users (1.48 per 1000 PY). The statin users had a significantly lower liver cancer mortality in univariate analysis [HR, 0.19; 95% confidence interval (CI), 0.06–0.61;
Fig. 1,
Table 2] and in age- and sex-adjusted analysis (HR, 0.20; 95% CI, 0.06–0.62). Hypercholesterolemia was associated with a decreased risk of liver cancer mortality in univariate analysis (HR, 0.36; 95% CI, 0.20–0.67 for total cholesterol ≥240 mg/dL) and in age- and sex-adjusted analysis (HR, 0.37; 95% CI, 0.20–0.69 for total cholesterol ≥240 mg/dL). High BMI (≥30 kg/m
2; HR, 2.10; 95% CI, 1.04–4.21) was also associated with an increased risk of liver cancer mortality in the age- and sex-adjusted analysis.
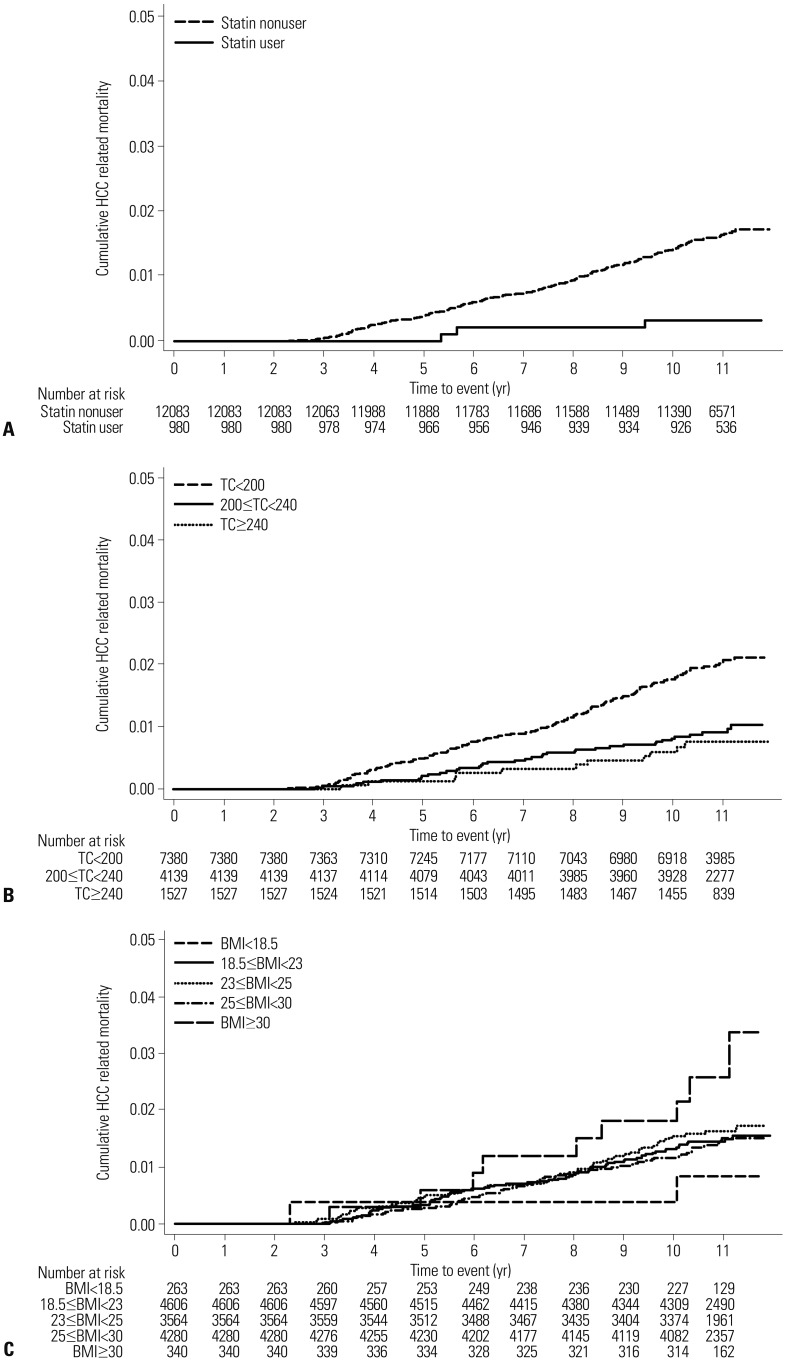 | Fig. 1Cumulative liver cancer mortality according to (A) statin use, (B) total cholesterol (TC), and (C) body mass index (BMI). HCC, hepatocellular carcinoma.
|
Table 2
Liver Cancer Mortality in Unadjusted and Adjusted Analyses
|
Mortality rate per 1000 PY (95% CI) |
Univariate analysis [HR (95% CI)] |
Age- and sex-adjusted analysis [HR (95% CI)] |
Multivariable analysis [HR (95% CI)] |
|
Statin |
|
|
|
|
|
No |
1.48 (1.28–1.71) |
Reference |
Reference |
Reference |
|
Yes |
0.29 (0.09–0.89) |
0.19 (0.06–0.61) |
0.20 (0.06–0.62) |
0.17 (0.04–0.70) |
|
BMI (kg/m2) |
|
|
|
|
|
<18.5 |
0.74 (0.19–2.97) |
0.56 (0.14–2.27) |
0.50 (0.12–2.05) |
0.28 (0.04–2.02) |
|
18.5–22.9 |
1.35 (1.06–1.72) |
Reference |
Reference |
Reference |
|
23–24.9 |
1.50 (1.16–1.95) |
1.11 (0.78–1.58) |
1.02 (0.71–1.45) |
1.06 (0.71–1.59) |
|
25–29.9 |
1.29 (1.00–1.67) |
0.95 (0.67–1.35) |
0.90 (0.63–1.28) |
0.96 (0.64–1.42) |
|
≥30 |
2.53 (1.32–4.86) |
1.88 (0.94–3.77) |
2.10 (1.04–4.21) |
2.46 (1.20–5.06) |
|
Total cholesterol (mg/dL) |
|
|
|
|
|
<200 |
1.84 (1.56–2.17) |
Reference |
Reference |
Reference |
|
200–239 |
0.86 (0.63–1.18) |
0.47 (0.33–0.67) |
0.48 (0.34–0.69) |
0.50 (0.34–0.74) |
|
≥240 |
0.67 (0.37–1.22) |
0.36 (0.20–0.67) |
0.37 (0.20–0.69) |
0.46 (0.24–0.88) |

Multivariable analysis after adjusting for demographic and metabolic factors showed consistent results. Statin use (HR, 0.17; 95% CI, 0.04–0.70) and hypercholesterolemia (HR, 0.46; 95% CI, 0.24–0.88 for total cholesterol ≥240 mg/dL) were associated with a decreased risk of liver cancer mortality (
Table 2). High BMI (≥30 kg/m
2; HR, 2.46; 95% CI, 1.20–5.06) was associated with an increased risk of liver cancer mortality.
The effect of statin use on liver cancer mortality among non-cirrhotic patients with CHB was investigated in this study, with a consideration paid to hypercholesterolemia and obesity. The results showed that statin use was associated with decreased liver cancer mortality when adjusting for cholesterol levels and BMI. The study also found that hypercholesterolemia was independently associated with decreased liver cancer mortality regardless of statin use.
A chemopreventive effect for statins has been reported in several studies. The anti-inflammatory, anti-angiogenic, and anti-tumor effects of statins can be related to a reduced risk of HCC development, and a lowered risk of HCC with statins has been reported worldwide.
6 A meta-analysis of 10 studies showed statin use was associated with a 37% reduced risk of HCC.
13 In addition, a recent study of patients with CHB showed that statin use can achieve a 33–44% risk reduction in HCC development.
14 With the relatively short median follow-up of 4.6 years, the study was not able to exhibit a mortality benefit for statin use. With a longer follow-up of about 10 years, our study was able to show that statin use contributes to decreased liver cancer mortality. These findings may serve to justify the use of statins for chemoprevention in patients with CHB.
This study also found that hypercholesterolemia is associated with a mortality benefit regardless of statin use. This is in line with a previous study that found both statin use and hypercholesterolemia were associated with improved survival in patients with cirrhosis.
11 With serum cholesterol levels as a surrogate marker of hepatic synthetic function, high cholesterol levels may suggest better survival of liver-related disease.
11 The better survival shown among statin users may have been from hypercholesterolemia and independent of any pharmacologic effect of statins. This study found that both statin use and hypercholesterolemia were independent factors associated with decreased liver cancer mortality. Thus, attention should be paid to both cholesterol levels and statin use when treating patients with CHB.
The association between BMI ≥30 kg/m
2 and increased liver cancer mortality documented in this study was consistent with a previous study.
15 A study conducted in Korea found a J-shaped pattern between BMI and death and showed the risk of death from cancer increased substantially among subjects with a BMI above 30.0 kg/m
2.
15 Other studies have also reported that obesity was associated with a higher risk of liver cancer development both in the general population and in patients with CHB.
1617
This study has some limitations. First, being a study based on observational data, this study may not be free of bias and confounding. As the NHIS of Korea does not incorporate serum HBV DNA levels or detailed data on liver cirrhosis status, neither was available for this study. In addition, the information on liver cancer incidence was not available in the given dataset. Second, although the impact of antiviral treatment, such as nucleos(t)ide analogues, on liver cancer mortality is significant, this study was not able to consider it, as data on the status of antiviral treatment in the study patients was not available. Third, cardiovascular mortality, which may have affected liver cancer mortality, was not taken into account as a competing risk. Moreover, this study was not able to include multiple co-morbidities that might affect the prognosis of patients. Lastly, the study population consisted exclusively of Koreans, which limits the generalizability of the findings. All Koreans are infected by genotype C HBV,
18 which is mostly acquired through vertical transmission and is associated with a high risk of disease progression.
In this nationwide retrospective cohort of non-cirrhotic patients with CHB, we found that hypercholesterolemia and BMI ≥30 kg/m2 were associated with liver cancer mortality. Moreover, statin use was found to be an independent predictor of decreased liver cancer mortality, regardless of cholesterol levels and BMI.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download