Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | Fig. 1Effects of propofol on hypoxia and reoxygenation (H/R)-induced cytotoxicity to HK-2 cells. Cell viability of the control group with neither H/R injury nor propofol pretreatment was used as a 100% benchmark. H/R injury led to reduced cell viability (##p<0.01 against the control group), and propofol pretreatment alleviated decreases in cell viability induced by H/R injury (*p<0.05 and **p<0.01 against the H/R injury group). No significant difference was observed between pretreatments with 50 µM and 100 µM propofol (Pro 10, 10 µM; Pro 25, 25 µM; Pro 50, 50 µM; Pro 100, 100 µM). |
 | Fig. 2Effects of propofol on LDH leakage in HK-2 cells. LDH levels were measured for the same control and experimental groups as in Fig. 1. Consistently, hypoxia and reoxygenation (H/R) injury led to significantly higher levels of LDH (##p<0.01 against the control group), and propofol pretreatment alleviated increases in LDH caused by H/R injury (**p<0.01 against H/R group). |
 | Fig. 3Effects of propofol on apoptosis of HK-2 cells. Flow cytometry was conducted on the same control and experimental groups as in Fig. 1 (A: Control group; B: Hypoxia and reoxygenation (H/R) group; C: Pro 10 group; D: Pro 25 group; E: Pro 50 group; F: Pro 100 group). (G) Flow cytometric analysis for (A–F) was carried out as described in the Materials and Methods section. H/R injury increased the number of apoptotic cells (##p<0.01 against the control group), and propofol pretreatment alleviated the observed increases (*p<0.05 and **p<0.01 against H/R group). |
 | Fig. 4Potential synergistic effect of propofol and SP600125 on alleviating cell apoptosis induced by hypoxia and reoxygenation (H/R) injury. (A–E) Representative figures of flow cytometry results for (A) the control group with neither H/R injury nor propofol/SP600125 pretreatment, (B) the H/R injury group with no pretreatment, (C) the SP600125 group pretreated with 10 µM SP600125 prior to H/R injury, (D) the Pro 50 group pretreated with 50 µM propofol prior to H/R injury, and (E) the Pro 50+SP600125 group pretreated with a combination of 50 µM propofol and 10 µM SP600125 prior to H/R injury. (F) Flow cytometric analysis for (A–E) was carried out as described in the Materials and Methods section. H/R injury increased cell apoptosis significantly (##p<0.01 against the control group), while a combination of propofol and SP600125 attenuated the increase in a potentially synergistic manner (*p<0.05 against H/R group; **p<0.01 against H/R group; ▲p<0.05 against Pro 50 group). |
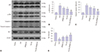 | Fig. 5Effects of propofol and SP600125 on the protein expression levels of JNK, p-JNK, caspase-3, cleaved-caspase-3, and Bcl-2 in HK-2 cells. Western blots were performed for the same control and experimental groups as in Fig. 4 to measure expression levels of the above-mentioned proteins. (A) Raw data. (B–D) Hypoxia and reoxygenation (H/R) injury increased the protein expression levels of p-JNK and cleaved-caspase-3, and decreased those of Bcl-2. Both SP600125 and propofol attenuated the changes in expression levels, and a combination of both alleviated the changes further (##p<0.01 against the control group; *p<0.05 and **p<0.01 against the H/R group; ▲p<0.05 and ▲▲p<0.01 against the Pro 50 group). |
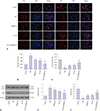 | Fig. 6Effects of propofol and SP600125 on autophagy induced by hypoxia and reoxygenation (H/R) injury. The same control and experimental groups as in Fig. 4 were subjected to fluorescence microscopy (A–C) and Western blots (D–F) to measure the protein expression levels of LC3II and p62 (autophagy markers). Both techniques showed that H/R injury increased the protein expression levels of LC3II and decreased those of p62. Both SP600125 and propofol attenuated the changes, and their alleviating effects were stronger when applied together (##p<0.01 against the control group; *p<0.05 and **p<0.01 against the H/R group; ▲p<0.05 against the Pro 50 group). |
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Huaxin Wang.
Data curation: Huaxin Wang and Xuan Peng.
Formal analysis: Yeda Xiao and Xuan Peng.
Funding acquisition: Huaxin Wang.
Investigation: Zhuo Wang.
Methodology: Yayi Huang and Xuan Peng.
Project administration: Huaxin Wang and Liying Zhan.
Resources: Huaxin Wang.
Software: Yeda Xiao.
Supervision: Liying Zhan.
Validation: Huaxin Wang and Liying Zhan.
Visualization: Yayi Huang and Zhuo Wang.
Writing—original draft: Huaxin Wang, Yayi Huang, and Zhuo Wang.
Writing—review & editing: Huaxin Wang and Liying Zhan.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download