1. Jürimäe J. Interpretation and application of bone turnover markers in children and adolescents. Curr Opin Pediatr. 2010; 22:494–500. PMID:
20508524.

2. Barera G, Beccio S, Proverbio MC, Mora S. Longitudinal changes in bone metabolism and bone mineral content in children with celiac disease during consumption of a gluten-free diet. Am J Clin Nutr. 2004; 79:148–154. PMID:
14684411.

3. Okabe R, Nakatsuka K, Inaba M, Miki T, Naka H, Masaki H, et al. Clinical evaluation of the Elecsys beta-CrossLaps serum assay, a new assay for degradation products of type I collagen C-telopeptides. Clin Chem. 2001; 47:1410–1414. PMID:
11468230.
4. Khadka B, Tiwari ML, Gautam R, Timalsina B, Pathak NP, Kharel K, et al. Correlates of biochemical markers of bone turnover among post-menopausal women. JNMA J Nepal Med Assoc. 2018; 56:754–758. PMID:
30387463.
5. Szulc P, Seeman E, Delmas PD. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int. 2000; 11:281–294. PMID:
10928217.

6. Clinical and Laboratory Standards Institute. Evaluation of precision of quantitative measurement procedures; approved guideline. 3rd ed. EP05-A3. Wayne (PA): Clinical and Laboratory Standards Institute;2014.
7. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. EP06-A. Wayne (PA): Clinical and Laboratory Standards Institute;2003.
8. Reed AH, Henry RJ, Mason WB. Influence of statistical method used on the resulting estimate of normal range. Clin Chem. 1971; 17:275–284. PMID:
5552364.

9. Clinical and Laboratory Standards Institute. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. 3rd ed. C28-A3. Wayne (PA): Clinical and Laboratory Standards Institute;2008.
10. Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference ranges. Clin Chem. 1990; 36:265–270. PMID:
2302771.

11. Bayer M. Reference values of osteocalcin and procollagen type I N-propeptide plasma levels in a healthy Central European population aged 0-18 years. Osteoporos Int. 2014; 25:729–736. PMID:
23974858.
12. Wolthers OD, Heuck C, Heickendorff L. Diurnal variations in serum and urine markers of type I and type III collagen turnover in children. Clin Chem. 2001; 47:1721–1722. PMID:
11514416.

13. Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, Trenti T, et al. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med. 2011; 49:1271–1274. PMID:
21605012.

14. Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996; 17:333–368. PMID:
8854049.

15. Akesson K. Biochemical markers of bone turnover. A review. Acta Orthop Scand. 1995; 66:376–386. PMID:
7676832.
16. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996; 382:448–452. PMID:
8684484.

17. Tobiume H, Kanzaki S, Hida S, Ono T, Moriwake T, Yamauchi S, et al. Serum bone alkaline phosphatase isoenzyme levels in normal children and children with growth hormone (GH) deficiency: a potential marker for bone formation and response to GH therapy. J Clin Endocrinol Metab. 1997; 82:2056–2061. PMID:
9215272.

18. Nielsen HK, Jørgensen JO, Brixen K, Møller N, Charles P, Christensen JS. 24-h profile of serum osteocalcin in growth hormone (GH) deficient patients with and without GH treatment. Growth Regul. 1991; 1:153–159. PMID:
1842347.
19. Heuck C, Skjaerbaek C, Wolthers OD. Diurnal rhythm of serum osteocalcin in normal children. Acta Paediatr. 1998; 87:930–932. PMID:
9764885.

20. Banfi G, Daverio R. In vitro stability of osteocalcin. Clin Chem. 1994; 40:833–834.

21. Garnero P, Grimaux M, Seguin P, Delmas PD. Characterization of immunoreactive forms of human osteocalcin generated in vivo and in vitro. J Bone Miner Res. 1994; 9:255–264. PMID:
8140939.

22. Power MJ, O’Dwyer B, Breen E, Fottrell PF. Osteocalcin concentrations in plasma prepared with different anticoagulants. Clin Chem. 1991; 37:281–284. PMID:
1993340.

23. Blumsohn A, Naylor KE, Timm W, Eagleton AC, Hannon RA, Eastell R. Absence of marked seasonal change in bone turnover: a longitudinal and multicenter cross-sectional study. J Bone Miner Res. 2003; 18:1274–1281. PMID:
12854838.

24. Schou AJ, Heuck C, Wolthers OD. Vitamin D supplementation to healthy children does not affect serum osteocalcin or markers of type I collagen turnover. Acta Paediatr. 2003; 92:797–801. PMID:
12892157.

25. Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone. 2002; 30:886–890. PMID:
12052458.

26. Huang Y, Eapen E, Steele S, Grey V. Establishment of reference intervals for bone markers in children and adolescents. Clin Biochem. 2011; 44:771–778. PMID:
21531216.

27. Horn PS, Pesce AJ, Copeland BE. A robust approach to reference interval estimation and evaluation. Clin Chem. 1998; 44:622–631. PMID:
9510871.

28. Yang L, Grey V. Pediatric reference intervals for bone markers. Clin Biochem. 2006; 39:561–568. PMID:
16423337.

29. Albertsson-Wikland K, Rosberg S, Karlberg J, Groth T. Analysis of 24-hour growth hormone profiles in healthy boys and girls of normal stature: relation to puberty. J Clin Endocrinol Metab. 1994; 78:1195–1201. PMID:
8175978.

30. Klein KO, Martha PM Jr, Blizzard RM, Herbst T, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. II. Estrogen levels as determined by an ultrasensitive bioassay. J Clin Endocrinol Metab. 1996; 81:3203–3207. PMID:
8784070.

31. Koivula MK, Risteli L, Risteli J. Measurement of aminoterminal propeptide of type I procollagen (PINP) in serum. Clin Biochem. 2012; 45:920–927. PMID:
22480789.

32. Garnero P, Vergnaud P, Hoyle N. Evaluation of a fully automated serum assay for total N-terminal propeptide of type I collagen in postmenopausal osteoporosis. Clin Chem. 2008; 54:188–196. PMID:
17998267.

33. Eastell R, Garnero P, Audebert C, Cahall DL. Reference intervals of bone turnover markers in healthy premenopausal women: results from a cross-sectional European study. Bone. 2012; 50:1141–1147. PMID:
22348982.

34. Yoo JI, Park AJ, Lim YK, Kweon OJ, Choi JH, Do JH, et al. Age-related reference intervals for total collagen-I-N-terminal propeptide in healthy Korean population. J Bone Metab. 2018; 25:235–241. PMID:
30574468.

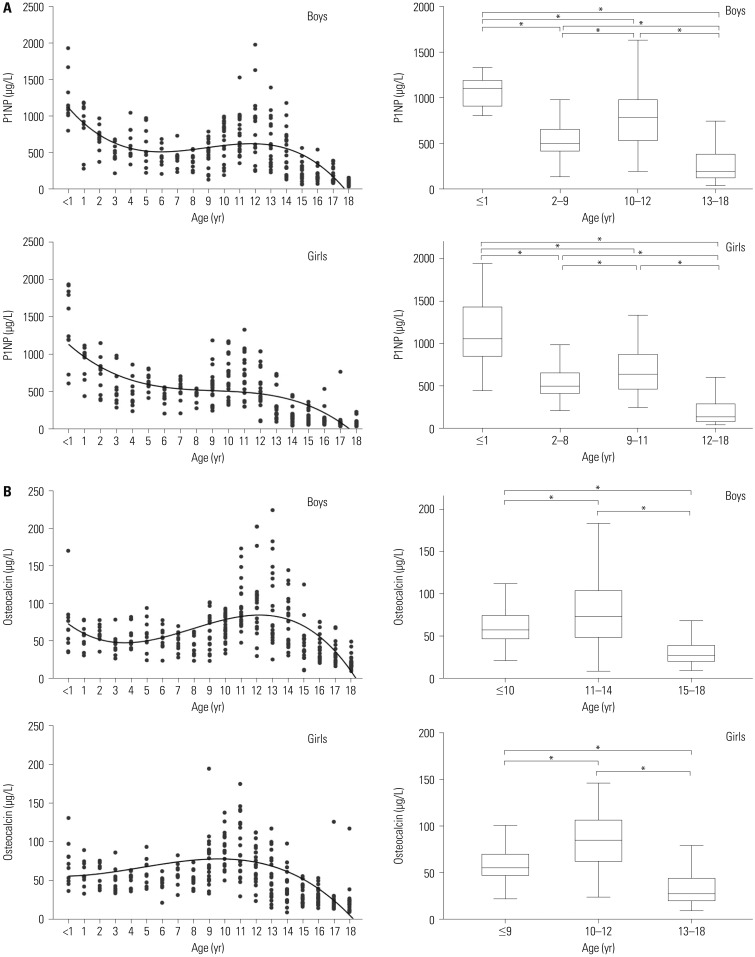
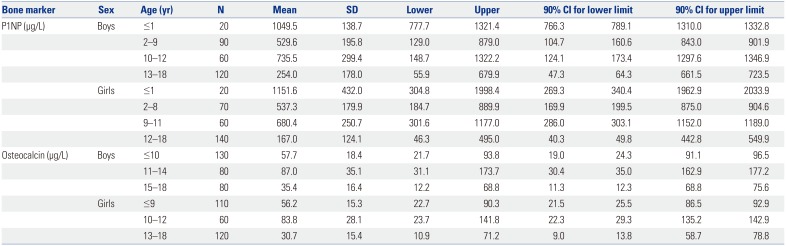




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download