Abstract
Purpose
To present differences in visual acuity and macular structure before and after surgery in patients with idiopathic epiretinal membrane (ERM) according to the presence of retinoschisis.
Methods
This retrospective observational study included 324 eyes with idiopathic ERM, that underwent pars plana vitrectomy with ERM and internal limiting membrane peeling, and were followed for more than 6 months. Subjects were classified into two groups according to the presence of retinoschisis using preoperative optical coherence tomography (OCT; group 1, ERM with retinoschisis; group 2, ERM without retinoschisis). Preoperative and postoperative macular structure changes and surgical outcomes were compared.
Results
Group 1 included 61 eyes, and group 2 included 263 eyes. Group 1 had a significantly higher preoperative and final postoperative best-corrected visual acuity compared with group 2 (p = 0.01, p = 0.02). Preoperative disorganization of retinal inner layers (DRIL) was significantly less in group 1 than group 2 (p = 0.01). Preoperative central macular thickness was not significantly different between the two groups. However, postoperative central macular thickness was significantly lower in group 1 than group 2 (p = 0.02, p = 0.01, p < 0.01). The ratio of the inner or outer layer in the total retinal thickness before surgery was significantly smaller in group 1 than in group 2 (p = 0.02, p = 0.04).
Conclusions
Preoperative visual acuity was better and the occurrence of DRIL was less in idiopathic ERM with retinoschisis than without retinoschisis. Postoperative visual and structural outcome was better in idiopathic ERM with retinoschisis than without retinoschisis. Retinoschisis may have played a role in reducing the tractional force given to the inner and outer retina.
Figures and Tables
 | Figure 1Representative group 2 (A, right eye, epiretinal membrane without retinoschisis) and group 1 (B, left eye, epiretinal membrane with retinoschisis) spectral-domain optical coherence tomography images in a 56-year-old woman. Note the presence of disorganization of retinal inner layers (DRIL, yellow arrows) (A). The retinoschisis is located between inner nuclear layer and outer plexiform layer red arrow heads (B). |
 | Figure 2Segmentation of inner retina (IR) and outer retina (OR) with epiretinal membrane. The mean IR and OR thickness was measured at the upper, lower, nasal, and temporal sides, 500 µm away from the fovea center. |
 | Figure 3Mean BCVA changes at baseline (preoperative) and at 1, 3, 6 months postoperatively and at the final visit for ERM patients with retinoschisis (group 1) or without retinoschisis (group 2). BCVA = best corrected visual acuity; logMAR = logarithm of minimal angle of resolution; ERM = epiretinal membrane; M = month(s); preop = preoperative; postop = postoperative. *p < 0.05 compared to baseline BCVA. |
 | Figure 4Mean CFT changes at baseline (preoperative) and at 1, 3, 6 months postoperatively and at the final visit for ERM patients with retinoschisis (group 1) or without retinoschisis (group 2). CFT = central foveal thickness; ERM = epiretinal membrane; M = month(s); preop = preoperative; postop = postoperative. *p < 0.05 compared to baseline CFT. |
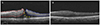 | Figure 5Spectral domain optical coherence tomography (SD-OCT) image changes after surgery of the left eye in a 65-year-old man. (A) Preoperative SD-OCT images with visual acuity logMAR 0.40. The inner retina at the nasal side (red outline) is relatively thickened, whereas the inner retina at the temporal side (blue outline) is preserved due to presence of retinoschisis (yellow arrowheads). (B) SD-OCT image at 12 months after operation with visual acuity logMAR 0.05. The thickness of inner retina at nasal side is not completely recovered compared with the temporal side of inner retina. |
Table 2
Comparisons of preoperative best corrected visual acuity and optical coherence tomography measurements of the two groups

Values are presented as mean ± standard deviation or number (%) unless otherwise indicated.
BCVA = best corrected visual acuity; logMAR = logarithm of minimal angle of resolution; CFT = central foveal thickness; IR = inner retina; OR = outer retina; DRIL = disorganization of retinal inner layers; IS/OS = inner segment/outer segment layer; OPL = outer plexiform layer; ONL = outer nuclear layer; INL = inner nuclear layer.
*Idiopathic ERM with retinoschisis; †idiopathic ERM without retinoschisis; ‡t-test.
References
1. Sidd RJ, Fine SL, Owens SL, Patz A. Idiopathic preretinal gliosis. Am J Ophthalmol. 1982; 94:44–48.
2. Pearlstone AD. The incidence of idiopathic preretinal macular gliosis. Ann Ophthalmol. 1985; 17:378–380.
3. Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997; 104:1033–1040.
4. Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994; 92:403–430.
5. Miliatos I, Lindgren G. Epiretinal membrane surgery evaluated by subjective outcome. Acta Ophthalmol. 2017; 95:52–59.
6. Margherio RR, Cox MS Jr, Trese MT, et al. Removal of epimacular membranes. Ophthalmology. 1985; 92:1075–1083.

7. Rice TA, De Bustros S, Michels RG, et al. Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology. 1986; 93:602–610.
8. de Bustros S, Thompson JT, Michels RG, et al. Vitrectomy for idiopathic epiretinal membranes causing macular pucker. Br J Ophthalmol. 1988; 72:692–695.

9. Greven CM, Slusher MM, Weaver RG. Epiretinal membrane release and posterior vitreous detachment. Ophthalmology. 1988; 95:902–905.
10. Asaria R, Garnham L, Gregor ZJ, Sloper JJ. A prospective study of binocular visual function before and after successful surgery to remove a unilateral epiretinal membrane. Ophthalmology. 2008; 115:1930–1937.

11. Pournaras CJ, Emarah A, Petropoulos IK. Idiopathic macular epiretinal membrane surgery and ILM peeling: anatomical and functional outcomes. Semin Ophthalmol. 2011; 26:42–46.
12. Donati S, Caprani SM, Semeraro F, et al. Morphological and functional retinal assessment in epiretinal membrane surgery. Semin Ophthalmol. 2017; 32:751–758.

13. Sandali O, El Sanharawi M, Basli E, et al. Epiretinal membrane recurrence: incidence, characteristics, evolution, and preventive and risk factors. Retina. 2013; 33:2032–2038.
14. Sheales MP, Kingston ZS, Essex RW. Associations between preoperative OCT parameters and visual outcome 3 months postoperatively in patients undergoing vitrectomy for idiopathic epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2016; 254:1909–1917.
15. Mitamura Y, Hirano K, Baba T, Yamamoto S. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. Br J Ophthalmol. 2009; 93:171–175.
16. Inoue M, Morita S, Watanabe Y, et al. Preoperative inner segment/outer segment junction in spectral-domain optical coherence tomography as a prognostic factor in epiretinal membrane surgery. Retina. 2011; 31:1366–1372.

17. Shimozono M, Oishi A, Hata M, et al. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am J Ophthalmol. 2012; 153:698–704.e1.
18. Lee EK, Yu HG. Ganglion cell-inner plexiform layer thickness after epiretinal membrane surgery: a spectral-domain optical coherence tomography study. Ophthalmology. 2014; 121:1579–1587.
19. Okamoto F, Sugiura Y, Okamoto Y, et al. Inner nuclear layer thickness as a prognostic factor for metamorphopsia after epiretinal membrane surgery. Retina. 2015; 35:2107–2114.
20. Lee JY, Kim HC. Ganglion cell layer thickness after anti-vascular endothelial growth factor treatment in retinal vein occlusion. J Korean Ophthalmol Soc. 2016; 57:63–70.

21. Arichika S, Hangai M, Yoshimura N. Correlation between thickening of the inner and outer retina and visual acuity in patients with epiretinal membrane. Retina. 2010; 30:503–508.
22. Koo HC, Rhim WI, Lee EK. Morphologic and functional association of retinal layers beneath the epiretinal membrane with spectral-domain optical coherence tomography in eyes without photoreceptor abnormality. Graefes Arch clin Exp Ophthalmol. 2012; 250:491–498.

23. Shin HJ, Lee SH, Chung H, Kim HC. Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012; 250:61–70.
24. Hosoda Y, Ooto S, Hangai M, et al. Foveal photoreceptor deformation as a significant predictor of postoperative visual outcome in idiopathic epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2015; 56:6387–6393.

25. Ahn SJ, Ahn J, Woo SJ, Park KH. Photoreceptor change and visual outcome after idiopathic epiretinal membrane removal with or without additional internal limiting membrane peeling. Retina. 2014; 34:172–181.
26. Zur D, Iglicki M, Feldinger L, et al. Disorganization of retinal inner layers as a biomarker for idiopathic epiretinal membrane after macular surgery-the DREAM study. Am J Ophthalmol. 2018; 196:129–135.

27. Cho KH, Park SJ, Cho JH, et al. Inner-retinal irregularity index predicts postoperative visual prognosis in idiopathic epiretinal membrane. Am J Ophthalmol. 2016; 168:139–149.
29. Vinores SA, Campochiaro PA, Conway BP. Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthalmol Vis Sci. 1990; 31:14–28.
30. Gupta P, Yee KM, Garcia P, et al. Vitreoschisis in macular diseases. Br J Ophthalmol. 2011; 95:376–380.

31. Hirose T. Retinoschisis. In : Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. 2nd ed. Philadelphia: WB Saunders;2000. v. 3:chap. 177.
32. de Bustros S, Rice TA, Michels RG, et al. Vitrectomy for macular pucker. Use after treatment of retinal tears or retinal detachment. Arch Ophthalmol. 1988; 106:758–760.
33. Kwon SI, Ko SJ, Park IW. The clinical course of the idiopathic epiretinal membrane after surgery. Korean J Ophthalmol. 2009; 23:249–252.
34. Kim J, Rhee KM, Woo SJ, et al. Long-term temporal changes of macular thickness and visual outcome after vitrectomy for idiopathic epiretinal membrane. Am J Ophthalmol. 2010; 150:701–709.e1.

35. Hwang DJ, Na KI, Kwon SI, Park IW. Long-term changes in visual acuity and foveal thickness after vitrectomy for idiopathic epiretinal membrane. J Korean Ophthalmol Soc. 2012; 53:434–439.
36. Yang HS, Kim JT, Joe SG, et al. Postoperative restoration of foveal inner retinal configuration in patients with epiretinal membrane and abnormally thick inner retina. Retina. 2015; 35:111–119.

37. Cho KH, Park SJ, Woo SJ, Park KH. Correlation between inner-retinal changes and outer-retinal damage in patients with idiopathic epiretinal membrane. Retina. 2018; 38:2327–2335.
38. Cacciamani A, Cosimi P, Di Nicola M, et al. Correlation between outer retinal thickening and retinal function impairment in patients with idiopathic epiretinal membranes. Retina. 2019; 39:331–338.

39. Hiscott PS, Grierson I, Trombetta CJ, et al. Retinal and epiretinal glia--an immunohistochemical study. Br J Ophthalmol. 1984; 68:698–707.
40. Kenawy N, Wong D, Stappler T, et al. Does the presence of an epiretinal membrane alter the cleavage plane during internal limiting membrane peeling? Ophthalmology. 2010; 117:320–323.e1.

41. Charteris DG, Downie J, Aylward GW, et al. Intraretinal and periretinal pathology in anterior proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 2007; 245:93–100.
42. McLeod D, Hiscott PS, Grierson I. Age-related cellular proliferation at the vitreoretinal juncture. Eye (Lond). 1987; 1(Pt 2):263–281.

43. Gupta P, Sadun AA, Sebag J. Multifocal retinal contraction in macular pucker analyzed by combined optical coherence tomography/scanning laser ophthalmoscopy. Retina. 2008; 28:447–452.
44. Yagi T, Sakata K, Funatsu H, et al. Macular microcirculation in patients with epiretinal membrane before and after surgery. Graefes Arch Clin Exp Ophthalmol. 2012; 250:931–934.

45. Yagi T, Sakata K, Funatsu H, Hori S. Evaluation of perifoveal capillary blood flow velocity before and after vitreous surgery for epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2012; 250:459–460.
46. Birol G, Wang S, Budzynski E, et al. Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol. 2007; 293:H1696–H1704.

47. Usui Y, Westenskow PD, Kurihara T, et al. Neurovascular crosstalk between interneurons and capillaries is required for vision. J Clin Invest. 2015; 125:2335–2346.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download