INTRODUCTION
MATERIALS AND METHODS
Patients
Isolation and ALI cultures of primary NECs
Purification and characterisation of PM2.5
Assessment of PM2.5 cytotoxicity to human cultured NECs
Assessment of the effect of PM2.5 on transepithelial resistance (TER) and paracellular flux in NECs established as ALI cultures
Quantitative real-time polymerase chain reaction (PCR) and immunostaining of TJs
Luminex and enzyme-linked immunosorbent assay (ELISA) analysis of cytokine concentration in culture medium
Statistical analysis
RESULTS
TJ expression in nasal mucosa harvested in different seasons
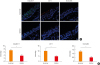 | Fig. 1(A) Representative images of immunofluorescent-stained claudin-1, ZO-1 and occludin in noninflammatory nasal mucosa collected during different seasons. The immunofluorescent staining for ZO-1 (red) and occludin (green) was mainly restricted to the apical compartment of epithelium, whereas the distribution of claudin-1 (green) was more extensive in the epithelium. A relatively weak staining was seen for claudin-1, occludin and ZO-1 in biopsy specimens collected during the winter compared to specimens collected during the summer. Bar = 20 μm. (B) Fluorescence intensities were quantified using ImageJ software (winter vs. summer; n = 7 and 5, respectively).
*Statistical significance at a level of P < 0.05.
|
Effect of PM2.5 on NECs established as ALI cultures
 | Fig. 2Effect of PM2.5 on the barrier function of ALI cultures established from NECs. (A) TER at different time points following exposure to medium alone (control), 50 μg/mL PM2.5 or 100 μg/mL PM2.5 intermittently. Due to some inherent variability in baseline TER measurements across samples, all measurements were normalized to a value of 1.0 at the beginning of the measurement (0 hour). (B) Dose-dependent increase in FITC-dextran paracellular flux in ALI cultures treated with different doses of PM2.5. Barrier integrity was determined by paracellular flux in response to PM2.5 treatment after 72 hours. (C) TER negatively correlates with FITC-dextran permeability in ALI cultures (exposed to 100 and 50 μg/mL PM2.5 or medium alone). Data are expressed as mean ± standard error of the mean, n = 12 donors per group in duplicate.PM2.5, particulate matter with an aerodynamic diameter of less than 2.5 μm; FITC, fluorescein isothiocyanate; ALI, air-liquid interface; TER, transepithelial resistance.
*P < 0.05; †P < 0.01; ‡P < 0.001.
|
Effect of pretreatment with budesonide on PM2.5-induced epithelial barrier damage
 | Fig. 3PM2.5 undermined the barrier integrity of air-liquid interface cultures established from nasal epithelial cells, which could not be prevented by budesonide pretreatment. The relative TER of cultures exposed to 50 μg/mL PM2.5 (A) or 100 μg/mL PM2.5 (B) and (C) paracellular flux of FITC-dextran 4kDa at 72 hours in cultures incubated in the absence or presence of 50/100 μg/mL PM2.5 was not significantly altered by pretreatment of the cultures for 1 hour with budesonide (1 μmol/L). Data are expressed as mean ± standard error of the mean, n = 5 donors per group in duplicate.PM2.5, particulate matter with an aerodynamic diameter less than 2.5 μm; FITC, fluorescein isothiocyanate; TER, transepithelial resistance.
*P < 0.05; †P < 0.001.
|
Effect of PM2.5 treatment on TJs mRNA and protein expression in ALI cultures established from NECs
 | Fig. 4Effect of PM2.5 on the expression of tight junction mRNAs in air-liquid interface cultures established from nasal epithelial cells. After incubation with PM2.5 for 72 hours, relative mRNA expression of claudin-1 was significantly decreased and expression of ZO-1 and claudin-7 significantly increased in cultures treated with 100 μg/mL PM2.5, compared to control cultures (n = 10 each for the control and 50-μg/mL PM2.5-treated groups, n = 9 for the 100-μg/mL PM2.5-treated group).PM2.5 with an aerodynamic diameter of less than 2.5 μm; mRNA, messenger RNA.
*P < 0.05, †P < 0.01.
|
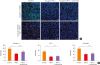 | Fig. 5(A) Representative images of claudin-1 (green), occludin (green) and ZO-1 (red) immunofluorescent staining in cultured nasal epithelial cells exposed to medium alone, 100 μg/mL PM2.5 or 100 μg/mL PM2.5 pretreated with budesonide. Cultures exposed to medium alone displayed distinct TJs at cell borders, whereas cultures treated with PM2.5 displayed diminished and less discernible TJs at cell-cell junctions. Cultures pre-treated with budesonide showed similar changes to those treated with just PM2.5. Representative images from 3 donors each are shown. Bar = 20 μm. (B) Fluorescence intensities were quantified using ImageJ software.PM2.5, particulate matter with an aerodynamic diameter less than 2.5 μm; N.S., not significant; TJ, tight junction; TRITC, tetramethylrhodamine.
*P < 0.05.
|
Effect of PM2.5 on inflammatory cytokines secreted by ALI cultures established from NECs
 | Fig. 6Cytokine concentrations (pg/mL) in ALI culture medium of NECs treated with medium alone, 50 μg/mL PM2.5 or 100 μg/mL PM2.5 intermittently for 3 days, respectively. Cultured NECs produced significantly more IL-8, TIMP-1 and TSLP after treatment with PM2.5, compared to cultures treated with medium alone. A dose-dependent effect was visible, although it was not statistically significant for all mediators. The secretion of other cytokines produced by NECs was not affected by PM2.5. Data are presented as mean ± standard error of the mean (n = 8 to 10 in each group).PM2.5, particulate matter with an aerodynamic diameter less than 2.5 μm; IL, interleukin; TIMP-1, TIMP metallopeptidase inhibitor 1; TSLP, thymic stromal lymphopoietin; MMP, matrix metalloproteinase; NEC, nasal epithelial cell.
*P < 0.05; †P < 0.01; ‡P < 0.001.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download