INTRODUCTION
MATERIALS AND METHODS
Animals
Experimental protocols for the treatment of the mice in each group
Collection of serum and measurement of serum levels of total and OVA-specific immunoglobulin E (IgE)
Differential cell counts in broncho-alveolar lavage (BAL) fluid
Buried-food pellet test
Histopathological examination
Immunofluorescence staining
Statistical analysis
RESULTS
 | Fig. 2Serum levels of (A) total IgE and (B) OVA-specific IgE.IgE, immunoglobulin E; OVA, ovalbumin; AR, allergic rhinitis; LAR, local allergic rhinitis; OD, olfactory disturbance.
*Statistically significant difference from the control group, P < 0.01; †Statistically significant difference from the AR group, P < 0.01; the Kruskal-Wallis and Mann-Whitney U tests.
|
 | Fig. 3Number of inflammatory cells (eosinophils, neutrophils, and lymphocytes) in the BAL fluid.BAL, broncho-alveolar lavage; AR, allergic rhinitis; LAR, local allergic rhinitis; OD, olfactory disturbance.
*Statistically significant difference from the control group, P < 0.05; †Statistically significant difference from the control group, P < 0.01; ‡Statistically significant difference from the AR group, P < 0.01; the Kruskal-Wallis and Mann-Whitney U tests.
|
 | Fig. 4Time until the hidden-food pellet detection as measured by the “buried-food pellet test.”NS, not significant; AR, allergic rhinitis; LAR, local allergic rhinitis; OD, olfactory disturbance.
|
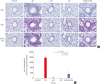 | Fig. 5Histopathological examination of the lung parenchyma (A) and the number of eosinophils that infiltrated into 1 mm2 of the lung parenchyma (B).AR, allergic rhinitis; LAR, local allergic rhinitis; OD, olfactory disturbance; H&E, hematoxylin and eosin; PAS, periodic acid Schiff.
*Statistically significant difference from the control group, P < 0.01; †Statistically significant difference from the control group, P < 0.001; ‡Statistically significant difference from the AR group, P < 0.001; the Kruskal-Wallis and Mann-Whitney U tests (scale bar = 200 μm).
|
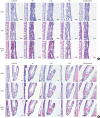 | Fig. 6Histopathological examination of the (A) nasal septa and (B) turbinate tissues (scale bar = 50 μm).AR, allergic rhinitis; LAR, local allergic rhinitis; OD, olfactory disturbance; H&E, hematoxylin and eosin; PAS, periodic acid Schiff.
|
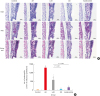 | Fig. 7Histopathological examination of the olfactory epithelia (A) and the number of eosinophils that infiltrated into 1 mm2 of the olfactory epithelia (“mucosa” is better. In sirius staining, positive cells are in submucosa area.) (B).AR, allergic rhinitis; LAR, local allergic rhinitis; OD, olfactory disturbance; H&E, hematoxylin and eosin; PAS, periodic acid Schiff.
*Statistically significant difference from the control group, P < 0.001; †Statistically significant difference from the AR group, P < 0.001; the Kruskal-Wallis and Mann-Whitney U tests (scale bar = 50 μm).
|
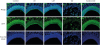 | Fig. 8Expression of OMP by immunofluorescent staining. FITC was used for the conjugation with the secondary antibody against anti-OMP antibody (green color).OMP, olfactory marker protein; FITC, fluorescein isothiocyanate; AR, allergic rhinitis; LAR, local allergic rhinitis; OD, olfactory disturbance.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download