Abstract
Overlap syndrome is defined as a disease entity that satisfies the classification criteria of at least two connective tissue diseases occurring concurrently or separately in a single patient. Here, we report a rare case of a 59-year-old woman with diffuse systemic sclerosis with lung involvement-rheumatoid arthritis overlap syndrome accompanied by cutaneous sarcoidosis. Although there is no consensus for the optimal treatment of overlap syndrome to date, this case of co-existing rheumatoid arthritis and systemic sclerosis with interstitial lung disease successfully responded to abatacept.
Systemic sclerosis (SSc) is a heterogeneous connective tissue disease (CTD) characterized by the triad of vascular injury, autoimmunity, and fibrosis of the skin and internal organs. SSc can occur in the form of overlap syndromes with other CTDs, including inflammatory myositis, rheumatoid arthritis (RA), Sjögren's syndrome (SS), and systemic lupus erythematosus (SLE). Sarcoidosis is a heterogeneous multisystemic inflammatory disease that is characterized by noncaseating granuloma in any organ system. In sarcoidosis, the skin is the second most commonly affected organ followed by the lung [1], and isolated cutaneous sarcoidosis is very rare. A few cases of sarcoidosis combined with RA, SSc, SS, SLE, or the spondyloarthropathies have been reported, but the coexistence of isolated cutaneous sarcoidosis and CTDs is rare.
Herein, we report a rare case of diffuse SSc (dSSc) in a patient who subsequently developed cutaneous sarcoidosis and RA.
A 53-year-old Korean woman visited our hospital with a subcutaneous nodule on the proximal part of the left forearm. The patient had been diagnosed with dSSc two years ago when she presented with Raynaud's phenomenon, sclerodactyly, digital pitting scars on the fingertip, and interstitial lung disease (ILD) in the bilateral lower lobes. Serology markers, including fluorescent antinuclear antibodie and anti-scl-70 antibodies, were positive. She fulfilled the preliminary classification criteria for SSc. The patient was treated with nifedipine for Raynaud's phenomenon in rheumatology department and completed one and half year of prednisolone treatment for SSc-ILD in pulmonology department. She had no longer any signs of progression of ILD without medication after SSc-ILD treatment.
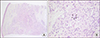
On physical examination, there was a solitary subcutaneous nodule on the left proximal forearm. She denied tenderness and itching on the subcutaneous nodule. A skin biopsy specimen from the left proximal forearm revealed chronic granulomatous inflammation with multinucleated giant cells in the dermis (Figure 1). The result of Tb/NTM real-time PCR with biopsy samples was negative. The pathologic finding was consistent with sarcoidosis. Additionally, the level of serum angiotensin converting enzyme (ACE) was elevated (67.9 U/L; reference, 7.5~53 U/L). On chest computed tomography (CT) scan and ophthalmological exam, there was no evidence of sarcoidosis systemic involvement. The patient was diagnosed with cutaneous sarcoidosis, and she did not receive any treatment because the lesion, which spontaneously regressed, was limited to the skin.
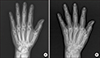
Two years later, she presented with swelling and tenderness in both knees, both wrists, and the right ankle. A laboratory examination revealed elevated C-reactive protein (CRP) (40.95 mg/L), and rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibody were highly positive (578 IU/mL and > 500 U/mL, respectively). A bone scan showed increased uptake in both shoulders, the right wrist, left knee, and both ankles. An ultrasound revealed joint effusions and synovial proliferation in both wrists and the right ankle. From these findings, she fulfilled the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA and was finally diagnosed with SSc-RA overlap syndrome. Initially, the arthritis had been well-controlled with bucillamine (200 mg per day) and low dose prednisolone (2.5~5 mg per day) for 3 years. However, a recent arthritis flare was not controlled well, and radiographic joint damage was found on plain hand radiographs (Figure 2). Thus, 6 mg/kg of abatacept with prednisolone (5 mg/day) was administered instead of conventional disease-modifying antirheumatic drugs. After the six-month treatment of abatacept, her disease activity score in 28 Joints decreased from 6.71 to 3.28, and her symptoms were relieved. In addition, ILD was followed regularly with high resolution CT scan, and has showed stable ILD since the abatacept infusion. The patient's final diagnosis was overlap syndrome with SSc-RA-subcutaneous sarcoidosis. Presently, she is followed in the outpatient clinic and has maintained a monthly abatacept infusion without an RA flare-up.
To the best of our knowledge, this is the first case of SSc-RA-cutaneous sarcoidosis overlap syndrome.
Connective tissue disease in overlap syndrome can occur simultaneously or sequentially. According to a review of case reports, the diagnosis of RA followed by SSc was more common than SSc followed by RA [2], although this is not the consistent pattern. The interval from the diagnosis of SSc to the diagnosis of RA ranged from 1 to 31 years in one report [3]. In our patient, RA developed five years after the diagnosis of SSc.
Since marked articular destruction and erosions are observed in patients with SSc-RA overlap syndrome [2], it is important to diagnose RA early in SSc patients. In our case, there was no obvious articular destruction or erosions at the time of RA diagnosis, but definite articular destruction and erosions rapidly progressed five years later (Figure 2). Therefore, it can be useful to evaluate CRP, RF, and anti-CCP antibody in SSc patients presenting with arthralgia or arthritis in order to diagnose possible RA-SSc overlap syndrome [3].
The clinical features observed in our patient were similar to those described in previous SSc-RA patient reports [23]. The most common organ involvement was erosive polyarthritis and pulmonary fibrosis, like our case, and patients with limited SSc were more affected in the great majority of overlap cases than those with diffused SSc [2].
Sarcoidosis, a multi-system granulomatous disorder of unclear etiology, can coexist with other CTDs, like SSc. To date, 30 cases of coexisting SSc and sarcoidosis have been reported [4]. The SSc-sarcoidosis overlap syndrome is common in females and patients with limited SSc, and approximately half of the cases (53%) were SSc followed by sarcoidosis whereas concurrent SSc and sarcoidosis occurred in 26%. Male patients with sarcoidosis-SSc overlap syndrome predominantly showed positive anti-Scl-70 (7 out of 9 cases), whereas most female patients with sarcoidosis-SSc overlap syndrome showed positive anti-centromere antibody (7 out of 8 cases). However, unlike systemic sarcoidosis, the coexistence of cutaneous sarcoidosis and CTD has rarely been reported [5]. Several previous reports describe patients who developed cutaneous sarcoidosis during the administration of etanercept, and rituximab in RA and microscopic polyangiitis patients, respectively [67]. These drugs were not used in our patient.
To the best of our knowledge, SSc-RA-cutaneous sarcoidosis overlap syndrome has not been reported thus far. Our patient suffered from subcutaneous sarcoidosis without systemic involvement. Subcutaneous sarcoidosis, first described by Darier and Roussy in 1904 [1], is a form of cutaneous sarcoidosis that involves the deep dermis and subcutaneous tissue [1]. The skin lesions of sarcoidosis are categorized into specific or nonspecific lesions depending on the presence or absence of typical noncaseating granulomas, respectively. The specific skin lesions can present in various forms: macules, papules, plaques, lupus pernio, nodules and ulcerations [1]. In our case, the skin lesion was a specific lesion and presented as a solitary subcutaneous nodule, and although multiple subcutaneous lesions are usually present, the nodule revealed sarcoidal granulomatous inflammation in adipose tissue of the skin [1]. Ando and colleagues [8] demonstrated that the incidence of an elevated ACE level was significantly higher in patients with subcutaneous sarcoidosis than in those with other types of cutaneous sarcoidosis, but mostly in the presence of a systemic disease component. Likewise, serum ACE was also elevated above the normal level in our case without systemic involvement.
The pathogeneses of sarcoidosis and SSc are not well known. It has been suggested that sarcoidosis is a Th1/Th17 multisystem disorder due to Th17 cells infiltrating the sarcoid lung and being driven by CCL20 chemokines during recruitment of the Th17 cell subset [9]. Ten Berge et al. [10] recently reported significantly increased proportions of circulating interleukin (IL)-17A+CD4+ T cells in patients with active sarcoidosis compared with healthy controls. Additionally, increased levels of IL-17 have been found in sarcoid granulomas and in the peripheral blood of patients with sarcoidosis, and it is believed to play a role in granuloma formation and maturation [10]. These findings suggest that Th17 cells may play a role in the pathogenesis of sarcoidosis.
Previous studies have shown that the pathogenesis of SSc involves the secretion of various cytokines by activated CD4+ T cells resulting in inflammation, vasculopathy, and fibrosis [11]. The role of the Th17-IL 17 axis in SSc has more recently been studied in vivo and in vitro. The number of Th17 cells and IL-17A are increased in the peripheral blood and skin of SSc patients [11], suggesting that IL-17A plays an essential role in SSc. However, serum IL-17 levels in SSc patients are decreased or similar to the levels in healthy controls [12]. The conflicting results may be explained by variations in sample size, the technique used for quantifying IL-17, or heterogeneity in the study populations. Nakashima et al. [13] suggested that IL-17A normally has an antifibrotic effect through reduction of collagen synthesis. On the contrary, IL-17A and Th17 cells in SSc patients promote fibroblast proliferation and collagen synthesis [11].
Many reports link IL-17 to the pathogenesis of RA. Levels of IL-17-producing Th17 cells are significantly increased in the peripheral blood and synovium of RA patients as compared with healthy controls [11]. Although the pathogenesis of sarcoidosis, SSc, and RA is not clear, it could be suggested that Th17/IL-17 may play a common role in coexisting sarcoidosis and SSc with RA.
Figures and Tables
References
2. Szücs G, Szekanecz Z, Zilahi E, Kapitány A, Baráth S, Szamosi S, et al. Systemic sclerosis-rheumatoid arthritis overlap syndrome: a unique combination of features suggests a distinct genetic, serological and clinical entity. Rheumatology (Oxford). 2007; 46:989–993.
3. Jinnin M, Ihn H, Yamane K, Asano Y, Yazawa N, Tamaki K. Clinical features of patients with systemic sclerosis accompanied by rheumatoid arthritis. Clin Exp Rheumatol. 2003; 21:91–94.
4. Senda S, Igawa K, Nishioka M, Murota H, Katayama I. Systemic sclerosis with sarcoidosis: case report and review of the published work. J Dermatol. 2014; 41:421–423.
5. Kato Y, Ohtsuka M, Yamamoto T. Cutaneous sarcoidosis concurrently involved in the sclerotic fingers of a patient with systemic sclerosis. J Dermatol. 2015; 42:331–333.

6. Vieira MA, Saraiva MI, Silva LK, Fraga RC, Kakizaki P, Valente NY. Development of exclusively cutaneous sarcoidosis in patient with rheumatoid arthritis during treatment with etanercept. Rev Assoc Med Bras (1992). 2016; 62:718–720.
7. Pescitelli L, Emmi G, Tripo L, Lazzeri L, Urban ML, Silvesri E, et al. Cutaneous sarcoidosis during rituximab treatment for microscopic polyangiitis: an uncommon adverse effect? Eur J Dermatol. 2017; 27:667–668.

8. Ando M, Miyazaki E, Hatano Y, Nishio S, Torigoe C, Yamasue M, et al. Subcutaneous sarcoidosis: a clinical analysis of nine patients. Clin Rheumatol. 2016; 35:2277–2281.
9. Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011; 66:144–150.

10. Ten Berge B, Paats MS, Bergen IM, van den Blink B, Hoogsteden HC, Lambrecht BN, et al. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford). 2012; 51:37–46.
11. Liu M, Wu W, Sun X, Yang J, Xu J, Fu W, et al. New insights into CD4(+) T cell abnormalities in systemic sclerosis. Cytokine Growth Factor Rev. 2016; 28:31–36.

12. Mathian A, Parizot C, Dorgham K, Trad S, Arnaud L, Larsen M, et al. Activated and resting regulatory T cell exhaustion concurs with high levels of interleukin-22 expression in systemic sclerosis lesions. Ann Rheum Dis. 2012; 71:1227–1234.
13. Nakashima T, Jinnin M, Yamane K, Honda N, Kajihara I, Makino T, et al. Impaired IL-17 signaling pathway contributes to the increased collagen expression in scleroderma fibroblasts. J Immunol. 2012; 188:3573–3583.

14. Jani M, Hirani N, Matteson EL, Dixon WG. The safety of biologic therapies in RA-associated interstitial lung disease. Nat Rev Rheumatol. 2014; 10:284–294.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download