Abstract
Anti-interleukin 17A agent, secukinumab is remarkably effective for treating patients with ankylosing spondylitis. However, the main safety concern of secukinumab is an increased risk of infection. Generally, neurosyphilis occurs a few years after the primary syphilitic infection. Rare cases of progressing to neurosyphilis with a much lower latency were reported. We report a case of rapid progressive neurosyphilis involving hearing loss in both ears in a patient with ankylosing spondylitis who was treated with secukinumab.
Prior to the institution of penicillin, syphilis was a common disease and neurosyphilis could develop in the course of syphilis [1]. It was reported that about 20% to 30% of untreated syphilis patients did not clear T. pallidum from the central nervous system (CNS), but the incidence of neurosyphilis decreased and characteristics of the disease changed after use of penicillin [2].
Anti-interleukin (IL) 17A monoclonal antibody agent, secukinumab is highly effective in the treatment of ankylosing spondylitis (AS) and psoriasis. The main safety concern of secukinumab is increased opportunistic infection, and mucocutaneous candidiasis is the most frequent type; however, the majority of these events have not been difficult to manage [3].
In this case, we present an AS patient with rapid progressive neurosyphilis who was treated with secukinumab, and discuss the principal characteristics of this disease.
A 32-year old male patient was admitted with hearing impairment in both sides that started about 5 weeks earlier. He was diagnosed as AS 8 years before admission. The diagnosis was made on inflammatory low back pain, HLA B-27 positivity, limitation of motion in lumbar spine, and bilateral sacroiliitis more than grade 2 by modified New York Criteria. He received biologics, such as tumor necrosis factor (TNF) inhibitors (adalimumab for 16 months, infliximab for 20 months, golimumab for 3 months and then etanercept for 15 months) and IL-17A inhibitor (secukinumab for 10 months). He had a history of being treated with multiple anti-TNF monoclonal antibodies, and received the IL-17A monoclonal antibody, secukinumab since 1 year ago. Until hospitalization, disease activity of AS was controlled, c-reactive protein level and erythrocyte sedimentation rate were within the normal range and score of Bath Ankylosing Spondylitis Disease Activity Index was 2.61, using secukinumab. Six weeks before admission, he had a headache and posterior neck pain. He received a local injection in posterior neck and occipital area and nerve block treatment in greater occipital nerve and paravertebral nerve at a private clinic, but his symptom was not relieved. After 1 week, he visited the emergency room (ER) with sudden sensorineural hearing loss, and was treated with oral prednisone 60 mg for 3 days followed by a tapering dose. But no significant change was seen in his hearing loss. After 10 days, he revisited the ER with a diffuse macula-papular rash on his palm and anterior trunk (Figure 1).
On admission, he had an intermittent headache and hearing loss on both sides. A pure-tone audiogram showed bilateral sensorineuronal hearing loss with 80 decibels. Magnetic resonance imaging (MRI) showed diffuse leptomeningeal enhancement along the interpeduncular cistern of the mesencephalon (midbrain) forming a thin plaque and enhancement of the cranial nerve sheath together with (extending toward) inner ear (VII~VIII complex) (Figure 2A and 2B). Blood venereal disease research laboratory (VDRL) was positive (1:32) and Treponema haemagglutination was also positive (1>640). HIV testing was negative. Cerebrospinal fluid (CSF) contained 473/mm3 white blood cells (WBC) with one-third polymorphonuclear cells, a slightly elevated protein (1.32 g/L) with positive VDRL. The serological test was consistent with neurosyphilis. A careful questioning of the patients revealed that he had sex with prostitutes about 2 months prior to developing skin rash and after 1 month a painless genital ulcer formed and spontaneously disappeared. He was treated with ceftriaxone 2 g bid intravenously for 14 days. After the seventh day of treatment, the disease activity was reevaluated. CSF analysis showed a decreased WBC count (131/mm3) and protein (0.93 g/L), and his headache disappeared and previous meningeal enhancement in MRI improved (Figure 2C and 2D). Three months after treatment, his hearing was gradually restored in pure-tone audiogram with 60 decibels and VDRL titer of serum was decreased (1:2). He was able to talk by hearing aids.
This patient had presented hearing loss on both sides simultaneously with secondary syphilitic cutaneous lesions following the administration of secukinumab. The cranial nerve palsies in this case were both auditory and likely due to meningeal enhancement of 8th nerve sheath, as shown in MRI. Syphilis in this patient shows a more aggressive natural course that included a decreased latency period before the onset of neurosyphilis and increased severity of the clinical manifestation in the setting of secukinumab treatment.
To the knowledge of the authors, secondary syphilis was reported in two patients with AS who had receiving anti-TNF monoclonal antibody and neurosyphilis was reported in only one patient [456]. This case is the first report of neurosyphilis in patient with AS under anti-IL-17A monoclonal antibody, secukinumab. In the absence of other immune deterioration such as HIV infection, secukinumab as well as continuous use of biologics seems to have caused rapid progressive neurosyphilis development.
Patients with neurosyphilis are classified into general paresis, syphilitic meningitis, meningovascular, and tabetic forms, based on their mode of clinical presentation [7]. General paresis was the most common form, which manifests cognitive impairment and behavior changes. Syphilitic meningitis belongs to this case and was the second most common form, followed by involvement of the cranial nerve, mainly ocular and auditory. Meningovascular, which manifested as an ischemic event in the brain artery, and tabetic forms, which involved the spinal cord, showed similar incidence in neurosyphilis. While MRI may contribute to an anatomical lesion, neurosyphilis is still best classified by the nature of clinical manifestations [8].
T. pallidum invades to the CSF at a very early stage during the infection. Because the number of patients with neuroinvasion (based on a CSF study) exceeded the number that developed symptomatic neurosyphilis, early invasion of the CNS with T. pallidum may be cleared by an inflammatory response in most patients with syphilis, even with inadequate therapy [2]. It is reported that adaptive T cell immunity (T cells producing IFN-γ and IL-17) may contribute to the clearance of T. pallidum from the CSF [9,10]. Generally, neurosyphilis follows a few years after the primary infection, but rarely some patients have a much lower latency. The chancre and a maculopapular rash occurred with neurological manifestations at the same time in some immunocompromised patients including HIV [711]. It is reported that neurosyphilis usually develops a more fulminant course in HIV patients, but the course of the disease is more insidious in immunocompetent patients [1]. Similarly, our patient is also shown to have a rapid disease progression due to lack of IL-17.
We report a case of rapid progressive neurosyphilis in a patient with ankylosing spondylitis who was treating with secukinumab. This case suggests that patients who are receiving the secukinumab should be cautious about the syphilis infection. Further investigations associated between IL-17 and syphilis are needed in the future.
Figures and Tables
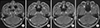 | Figure 2Magnetic resonance imaging (MRI) features of neurosyphilis during follow-up. MRI revealed diffuse leptomeningeal enhancement along the interpeduncular cistern of the mesencephalon (midbrain) forming a thin plaque (A) and enhancement of cranial nerve sheath together with (extending toward) inner ear (VII~VIII complex) (B) before treatment. MRI showed an improved lesion of previous meningeal enhancement (C, D) after the seventh day of treatment. |
Notes
References
1. Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004; 75:1727–1730.
2. Marra CM. Neurosyphilis. Continuum (Minneap Minn). 2015; 21(6 Neuroinfectious Disease):1714–1728.

3. Langley RG, Kimball AB, Nak H, Xu W, Pangallo B, Osuntokun OO, et al. Long-term safety profile of ixekizumab in patients with moderate-to-severe plaque psoriasis: an integrated analysis from 11 clinical trials. J Eur Acad Dermatol Venereol. 2019; 33:333–339.
4. Iglesias-Plaza A, Iglesias-Sancho M, Quintana-Codina M, García-Miguel J, Salleras-Redonnet M. Syphilis in the setting of anti-tumor necrosis factor alpha therapy. Reumatol Clin. 2018; 02. 03. DOI: 10.1016/j.reuma.2017.12.008. [Epub].

5. Bories-Haffner C, Buche S, Paccou J. Secondary syphilis occurring under anti-TNFalpha therapy. Joint Bone Spine. 2010; 77:364–365.
6. Assikar S, Doffoel-Hantz V, Sparsa A, Bonnetblanc JM. Early neurosyphilis with etanercept treatment. Eur J Dermatol. 2013; 23:901–902.

7. Drago F, Merlo G, Ciccarese G, Agnoletti AF, Cozzani E, Rebora A, et al. Changes in neurosyphilis presentation: a survey on 286 patients. J Eur Acad Dermatol Venereol. 2016; 30:1886–1900.
8. Nagappa M, Sinha S, Taly AB, Rao SL, Nagarathna S, Bindu PS, et al. Neurosyphilis: MRI features and their phenotypic correlation in a cohort of 35 patients from a tertiary care university hospital. Neuroradiology. 2013; 55:379–388.

9. Pastuszczak M, Jakiela B, Wielowieyska-Szybinska D, Jaworek AK, Zeman J, Wojas-Pelc A. Elevated cerebrospinal fluid interleukin-17A and interferon-γ levels in early asymptomatic neurosyphilis. Sex Transm Dis. 2013; 40:808–812.
10. Stary G, Klein I, Brüggen MC, Kohlhofer S, Brunner PM, Spazierer D, et al. Host defense mechanisms in secondary syphilitic lesions: a role for IFN-gamma-/IL-17-producing CD8+ T cells? Am J Pathol. 2010; 177:2421–2432.
11. Sadeghani K, Kallini JR, Khachemoune A. Neurosyphilis in a man with human immunodeficiency virus. J Clin Aesthet Dermatol. 2014; 7:35–40.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download