Abstract
Patients with severe active lupus nephritis (LN) require immunosuppressive therapy to induce remission. However, the development of profound hypogammaglobulinemia causing cytomegalovirus (CMV) disease is a rare occurrence during standard immunotherapy. A 27-year-old woman who presented with active LN along with moderate renal impairment was treated with of mycophenolate mofetil (MMF) and methylprednisolone. MMF was soon switched with low-dose intravenous (IV) cyclophosphamide (CYC) owing to the development of posterior reversible encephalopathy syndrome and deterioration of renal function requiring hemodialysis. After two cycles of IV CYC, she developed CMV colitis and pneumonia. Although her serum immunoglobulin (Ig) concentrations before receiving immunosuppressive treatment were normal, they were profoundly reduced at CMV disease onset and continued to maintain low level for 30 months. Severe hypogammaglobulinemia can occur during standard therapy for LN, especially in patients with impaired renal function, pointing out the importance of close monitoring of Ig levels and CMV infection.
Lupus nephritis (LN) is the most common and severe manifestation of systemic lupus erythematosus (SLE) associated with a high incidence of morbidity and mortality [1]. Guidelines recommend mycophenolate mofetil (MMF) (total daily oral dose of 2~3 g) or intravenous (IV) cyclophosphamide (CYC) along with glucocorticoids for induction treatment in International Society of Nephrology/Renal Pathology Society (ISN/RPS) class III/IV LN [23]. Cytomegalovirus (CMV) infection is a major complication in an immunocompromised host which primarily affects patients with acquired immunodeficiency syndrome (AIDS), organ transplant recipients or patients with autoimmune diseases receiving immunosuppressive therapy [4]. Hypogammaglobulinemia of <400 mg/dL has been identified as an important risk factor for infection in solid organ transplantation recipients with 2.4 times higher odds of developing CMV infections [5]. Although CMV infection is an emerging problem in active SLE patients receiving aggressive immunosuppression [6], profound hypogammaglobulinemia resulting in CMV infection during standard immunosuppressive treatment for LN has not been previously reported. Here, we report the first case of CMV infection associated with severe hypogammaglobulinemia during the treatment of active LN.
A 27-year-old woman was referred to our rheumatology clinic on September 8, 2015 owing to a complaint of generalized edema which insidiously developed over the past 2 months. She had gained 3 kg weight within a period of 2 months. On admission, she reported malaise, decreased urine output and oral ulcer, but denied having fever, photosensitivity, skin rash, or arthralgia. Her past medical history and family history were not significant. On examination, her body temperature was 37.1℃, blood pressure was 150/109 mmHg, and pulse rate was 98/min. Multiple shallow ulcers were observed on her hard palate. Lung sound was decreased on both lower lung fields. She had 3+ peripheral edema in her lower extremities. Examination findings for the skin, heart, abdomen, and joints were not remarkable. Laboratory examination showed white blood cell count of 3,520/µL; lymphocyte, 591/µL; hemoglobin, 9.9 g/dL; and platelet, 32×103/µL. Serum blood urea nitrogen (BUN) was 35 mg/dL; creatinine, 1.63 mg/dL; total protein, 3.9 mg/dL; and albumin, 1.9 g/dL. Urinalysis revealed 4+ for protein with hematuria and pyuria. Urine protein was found to be 7.26 g/24 hours. Liver function test result, erythrocyte sedimentation rate (ESR), and C-reactive protein level were normal. Antinuclear antibody (ANA) was positive at 1:640 titer with speckled pattern and anti-double-stranded DNA (anti-dsDNA) antibody was positive at a concentration of >200.0 IU/mL. Extractable nuclear antigen antibodies were positive for anti-SSA and anti-SSB but negative for anti-RNP and anti-Sm. Moreover, antiphospholipid antibodies were negative. Immunoglobulin (Ig) levels were within normal limits with values of IgG 1,020 mg/dL (reference range 700~1,600 mg/dL), IgA 227 mg/dL (reference range 70~400 mg/dL), and IgM 108 mg/dL (reference range 40~230 mg/dL). Complement (C) levels were markedly decreased with C3 19.2 mg/dL (reference range 90~180 mg/dL) and C4 3.3 mg/dL (reference range 10~40 mg/dL). Chest X-ray showed bilateral pleural effusion, and kidney biopsy results revealed LN ISN/RPS class IV-G (A). She was diagnosed with SLE along with active LN and was given induction treatment with methylprednisolone 1.0 g pulse therapy for 3 days followed by 3.0 g of MMF and a high-dose steroid therapy (62.5 mg of IV methylprednisolone).
Eight days after LN induction therapy, she developed generalized seizure and experienced a sudden elevation of blood pressure up to 200/110 mmHg and was diagnosed with posterior reversible encephalopathy syndrome (PRES). During the PRES episode, oliguria developed. Proteinuria increased to 14 g/day and renal impairment progressed to reach a creatinine level of 4.16 mg/dL, consequently requiring hemodialysis. To control her LN activity, MMF was changed to IV CYC 500 mg fortnightly Euro-lupus nephritis regimen [7]. After two cycles of IV CYC pulse therapy, her clinical parameters started to improve, and her creatinine level decreased to 1.59 mg/dL. However, 4 days before the initiation of the 3rd cycle of IV CYC, she reported fever, cough, and diarrhea. Laboratory examinations revealed white blood cell count of 1,910/µL; lymphocyte, 269/µL; hemoglobin, 9.9 g/dL; and platelet, 83×103/µL together with an increased CRP level (2.9 mg/dL). ESR was within the normal range with 8 mm/hr. Ig levels were markedly reduced as follows: IgG, 262 mg/dL; IgA, 46.7 mg/dL; and IgM, 19.6 mg/dL. Chest X-ray and computed tomography scan revealed diffuse reticulonodular opacities in both lungs. Plasma CMV DNA had increased to 53,004 copies/mL. CMV was also detected in bronchoalveolar lavage fluid samples and sigmoid colon biopsy. The third cycle of CYC was canceled due to the CMV infection involving lung and colon. She was treated with IV Ig for 5 days and with ganciclovir for 3 weeks until the resolution of CMV colitis and pneumonia. Although CMV DNA copies decreased to <40/mL 1 month after ganciclovir treatment, Ig levels remained persistently low (IgG 386 mg/dL, IgA 12.5 mg/dL, IgM <16.8 mg/dL) and maintained low level for 30 months. Treatment with MMF was restarted after the recovery of CMV infection. Unfortunately, she had an additional episode of CMV reactivation with fever and diarrhea 2 months after the initial ganciclovir treatment requiring further antiviral treatment and cessation of MMF. Tacrolimus was tried instead of MMF for the maintenance therapy but failed due to the development of tubulointerstitial nephritis. We started MMF 8 month after the last CMV infection and maintained it. At 3 years of follow-up, her disease activity was found to be in remission on 4 mg of methylprednisolone every other day and 300 mg of hydroxychloroquine daily. LN was also in complete remission together with full recovery of renal function. Her Ig levels were restored to the normal. Figure 1 shows her clinical course.
CMV infection primarily occurs as a self-limited febrile illness with an establishment of latency in various cells throughout the host's lifetime without significant reactivation [4]. However, in immunocompromised hosts, the virus may become reactivated and may cause devastating damage in multiple organs including the lung, gastrointestinal tract, liver, bone marrow, and retina [8]. Although CMV infection was infrequently reported in patients with rheumatic diseases, occurring in 151/7,377 (2%) of the patients studied, it was reportedly associated with fatal outcome. Among the rheumatic diseases, SLE was the most frequent cause accounting for 50% of CMV infections. Additionally, CMV infections in SLE resulted in high rates of mortality and morbidity among the infected individuals [9].
The risk factors for CMV reactivation in patients with SLE are not well studied. In organ transplant recipients, extrinsic factors such as intensity of immunosuppression, use of lymphocyte depleting drugs, critical illness, and host factors (such as age, leukopenia, and lymphopenia) are known risk factors for CMV reactivation [10]. Additionally, hypogammaglobulinemia is an important risk factor after organ transplantation [5]. Risk factors for CMV infection in SLE are assumed to be similar with those in organ transplant recipients. With the availability of more potent immunosuppressive drugs for SLE treatment, immunosuppressive treatment is an emerging factor contributing to an increased risk of CMV infections in patients with active SLE [6]. MMF, which is known to have fewer side effects compared with CYC, is now a key drug used in the treatment of ISN/RPS class III or IV LN [23]. Since humoral immunity is necessary for protection against CMV to control either primary dissemination or local viral spreading [11], severe CMV infections have been reported in cardiac transplant recipients who had hypogammaglobulinemia on MMF maintenance. Although hypogammaglobulinemia was reportedly associated with CMV infections in organ transplant recipients and in patients with immunodeficiency, this association has not been reported in patients with SLE. The reason for this may be related to the fact that hypergammaglobulinemia is common in active SLE. However, some patients were reported to have transient hypogammaglobulinemia especially after receiving cytotoxic drugs, but the causal relationship between hypogammaglobulinemia and the type and dose of the medication had not been documented [12]. In our patient, severe hypogammaglobulinemia occurred during the standard therapy for active LN. Intense immunosuppression involving switching of immunosuppressive drugs from MMF to CYC and high-dose MMF (3 gram per day) owing to critical disease condition may have contributed to the hypogammaglobulinemia development, resulting in CMV infection. Asian ethnicity and impaired renal function may also have contributed toward increasing drug toxicity, consistent with the findings reported in the ALMS study, i.e., serious adverse events were more prevalent among Asian patients receiving MMF [13].
CMV infections mimic lupus flare, causing confusion in diagnosis [14]. CMV can suppress bone marrow activity, thereby causing pancytopenia especially during severe infections, which is indistinguishable from hematologic manifestations of active SLE. Our patient also presented with fever and pancytopenia at the onset of CMV infection. Increase in CMV viral load associated with severe hypogammaglobulinemia of IgG level < 400 mg/dL provided clues for diagnosis. At present, serologic testing of CMV IgG is recommended for both recipients and donors before performing transplantation, especially solid organ and hematopoietic stem cell transplantations [15]. As exemplified by our case, for facilitating early diagnosis and prompt intervention, it may be advantageous to conduct serologic testing for CMV and IgG levels before subjecting active SLE patients, who require intense immunosuppression as in organ transplant recipients, to immunosuppression therapy.
Unpredictable opportunistic infections, such as that caused by CMV, associated with severe hypogammaglobulinemia can potentially occur in SLE patients during standard immunosuppressive therapy. It may be beneficial to monitor CMV serology and IgG levels before starting immunosuppressive treatment and at regular interval to reduce the risk of mortality and morbidity.
Figures and Tables
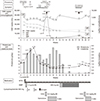 | Figure 1Clinical course of the SLE patient who presented CMV infection associated with severe hypogammaglobulinemia during standard immunosuppressive therapy. SLE: systemic lupus erythematosus, BP: blood pressure, HD: hemodialysis, CMV: cytomegalovirus, CT: computed tomography, BAL: bronchoalveolar lavage, POI: poor oral intake, Ig: immunoglobulin, MTS: methylprednisolone, MMF: mycophenolate mofetil, IVIG: intravenous immunoglobulin, Bid: twice a day. |
References
1. Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore). 2003; 82:299–308.
2. Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012; 64:797–808.
3. Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012; 71:1771–1782.
4. Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002; 34:1094–1097.
5. Mawhorter S, Yamani MH. Hypogammaglobulinemia and infection risk in solid organ transplant recipients. Curr Opin Organ Transplant. 2008; 13:581–585.

6. Berman N, Belmont HM. Disseminated cytomegalovirus infection complicating active treatment of systemic lupus erythematosus: an emerging problem. Lupus. 2017; 26:431–434.
7. Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, Garrido Ed, Danieli MG, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002; 46:2121–2131.

8. Koszinowski UH, Del Val M, Reddehase MJ. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990; 154:189–220.
9. Takizawa Y, Inokuma S, Tanaka Y, Saito K, Atsumi T, Hirakata M, et al. Clinical characteristics of cytomegalovirus infection in rheumatic diseases: multicentre survey in a large patient population. Rheumatology (Oxford). 2008; 47:1373–1378.

10. Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013; 96:333–360.
11. Wirtz N, Schader SI, Holtappels R, Simon CO, Lemmermann NA, Reddehase MJ, et al. Polyclonal cytomegalovirus-specific antibodies not only prevent virus dissemination from the portal of entry but also inhibit focal virus spread within target tissues. Med Microbiol Immunol. 2008; 197:151–158.

12. Cronin ME, Balow JE, Tsokos GC. Immunoglobulin deficiency in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 1989; 7:359–364.
13. Isenberg D, Appel GB, Contreras G, Dooley MA, Ginzler EM, Jayne D, et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology (Oxford). 2010; 49:128–140.

14. Zhang J, Dou Y, Zhong Z, Su J, Xu D, Tang F, et al. Clinical characteristics and therapy exploration of active human cytomegalovirus infection in 105 lupus patients. Lupus. 2014; 23:889–897.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download