Abstract
Objective
Acute anterior uveitis (AAU) is the most common extra-articular manifestation in patients with axial spondyloarthritis (axSpA). However, the relationship between AAU and radiographic progression in axSpA remains unclear. Hence, we investigated whether the presence of AAU is associated with radiographic structural damage in patients with axSpA.
Methods
Clinical and radiographic data were obtained from 253 patients with axSpA. Radiographic progression over 2 years was assessed using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). Progression was defined as mSASSS worsening by ≥ two units. Using propensity score (PS) matching, differences between patients with and without AAU were analyzed.
Results
The proportion of progressors among patients with AAU was lower than that of patients without AAU (13.6% vs. 29.5%, p=0.058). The rate of increase in mSASSS and number of syndesmophytes were lower in patients with AAU than patients without AAU (0.57±1.37 vs. 1.02±1.79, p=0.085 and 0.46±1.45 vs. 0.83±1.62, p=0.158). In multivariate regression analysis, presence of AAU was independently associated with slowed radiographic progression (odds ratio [95% confidence interval] 0.21 [0.07, 0.67], p=0.004).
Go to : 
REFERENCES
1. Sieper J, Braun J, Dougados M, Baeten D. Axial spondyloarthritis. Nat Rev Dis Primers. 2015; 1:15013.

3. Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl. 2006; 78:4–11.
4. Krüger K, von Hinüber U, Meier F, Tian H, Böhm K, Jugl SM, et al. Ankylosing spondylitis causes high burden to patients and the healthcare system: results from a German claims database analysis. Rheumatol Int. 2018; 38:2121–31.

5. Stolwijk C, Essers I, van Tubergen A, Boonen A, Bazelier MT, De Bruin ML, et al. The epidemiology of extra-articular manifestations in ankylosing spondylitis: a population-based matched cohort study. Ann Rheum Dis. 2015; 74:1373–8.

6. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015; 74:65–73.

7. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009; 68:777–83.

8. Gao X, Wendling D, Botteman MF, Carter JA, Rao S, Cifaldi M. Clinical and economic burden of extra-articular manifestations in ankylosing spondylitis patients treated with antitumor necrosis factor agents. J Med Econ. 2012; 15:1054–63.

9. Koo BS, Lim JW, Shin JH, Kim TH. Characteristics of uveitis in patients with ankylosing spondylitis in Korea: a single-center survey. J Rheum Dis. 2018; 25:28–33.

10. Moltó A, Paternotte S, Comet D, Thibout E, Rudwaleit M, Claudepierre P, et al. Performances of the Assessment of SpondyloArthritis International Society axial spondyloarthritis criteria for diagnostic and classification purposes in patients visiting a rheumatologist because of chronic back pain: results from a multicenter, cross-sectional study. Arthritis Care Res (Hoboken). 2013; 65:1472–81.
11. Chen CH, Lin KC, Chen HA, Liao HT, Liang TH, Wang HP, et al. Association of Acute Anterior uveitis with disease activity, functional ability and physical mobility in patients with ankylosing spondylitis: a cross-sectional study of Chinese patients in Taiwan. Clin Rheumatol. 2007; 26:953–7.

12. Lie E, Lindström U, Zverkova-Sandström T, Olsen IC, Forsblad-d'Elia H, Askling J, et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Ann Rheum Dis. 2017; 76:1515–21.

13. Deminger A, Klingberg E, Geijer M, Göthlin J, Hedberg M, Rehnberg E, et al. A five-year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res Ther. 2018; 20:162.

14. Atagunduz P, Aydin SZ, Bahadir C, Erer B, Direskeneli H. Determinants of early radiographic progression in ankylosing spondylitis. J Rheumatol. 2010; 37:2356–61.

15. Jeong H, Bea EK, Lee J, Koh EM, Cha HS. Body mass index and estrogen predict radiographic progression in the spine in ankylosing spondylitis. Joint Bone Spine. 2015; 82:473–4.

16. Kim TJ, Lee S, Joo KB, Park DJ, Park YW, Lee SS, et al. The presence of peripheral arthritis delays spinal radiographic progression in ankylosing spondylitis: observation Study of the Korean Spondyloarthropathy Registry. Rheumatology (Oxford). 2014; 53:1404–8.

17. Kim H, Lee J, Ahn JK, Hwang J, Park EJ, Jeong H, et al. Predictive factors of radiographic progression in ankylosing spondylitis. Korean J Intern Med. 2015; 30:391–7.

18. Johnson SR, Tomlinson GA, Hawker GA, Granton JT, Feldman BM. Propensity score methods for bias reduction in observational studies of treatment effect. Rheum Dis Clin North Am. 2018; 44:203–13.

19. Luo Z, Gardiner JC, Bradley CJ. Applying propensity score methods in medical research: pitfalls and prospects. Med Care Res Rev. 2010; 67:528–54.

20. Molto A, Gossec L, Meghnathi B, Landewé RBM, van der Heijde D, Atagunduz P, et al. An Assessment in SpondyloArthritis International Society (ASAS)-endorsed definition of clinically important worsening in axial spondyloarthritis based on ASDAS. Ann Rheum Dis. 2018; 77:124–7.

21. Creemers MC, Franssen MJ, van't Hof MA, Gribnau FW, van de Putte LB, van Riel PL. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis. 2005; 64:127–9.

22. Poddubnyy D, Conrad K, Haibel H, Syrbe U, Appel H, Braun J, et al. Elevated serum level of the vascular endothelial growth factor predicts radiographic spinal progression in patients with axial spondyloarthritis. Ann Rheum Dis. 2014; 73:2137–43.

23. Poddubnyy D, Haibel H, Listing J, Märker-Hermann E, Zeidler H, Braun J, et al. Baseline radiographic damage, elevated acutephase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum. 2012; 64:1388–98.

24. Poddubnyy D, Protopopov M, Haibel H, Braun J, Rudwaleit M, Sieper J. High disease activity according to the Ankylosing Spondylitis Disease Activity Score is associated with accelerated radiographic spinal progression in patients with early axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort. Ann Rheum Dis. 2016; 75:2114–8.

25. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984; 27:361–8.
26. MacKay K, Brophy S, Mack C, Doran M, Calin A. The development and validation of a radiographic grading system for the hip in ankylosing spondylitis: the bath ankylosing spondylitis radiology hip index. J Rheumatol. 2000; 27:2866–72.
28. Lee DK. Alternatives to P value: confidence interval and effect size. Korean J Anesthesiol. 2016; 69:555–62.

29. van Tubergen A, Ramiro S, van der Heijde D, Dougados M, Mielants H, Landewé R. Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Ann Rheum Dis. 2012; 71:518–23.

30. Poddubnyy D, Sieper J. Radiographic progression in ankylosing spondylitis/axial spondyloarthritis: how fast and how clinically meaningful? Curr Opin Rheumatol. 2012; 24:363–9.
31. Baeten D, Breban M, Lories R, Schett G, Sieper J. Are spon-dylarthritides related but distinct conditions or a single disease with a heterogeneous phenotype? Arthritis Rheum. 2013; 65:12–20.

32. Essers I, Ramiro S, Stolwijk C, Blaauw M, Landewé R, van der Heijde D, et al. Do extra-articular manifestations influence outcome in ankylosing spondylitis? 12-year results from OASIS. Clin Exp Rheumatol. 2016; 34:214–21.
33. Park JW, Kim MJ, Lee JS, Ha YJ, Park JK, Kang EH, et al. Impact of tumor necrosis factor inhibitor versus non-steroidal antiinflammatory drug treatment on radiographic progression in early ankylosing spondylitis: its relationship to inflammation control during treatment. Arthritis Rheumatol. 2019; 71:82–90.

34. Villaverde-García V, Cobo-Ibáñez T, Candelas-Rodríguez G, Seoane-Mato D, Campo-Fontecha PDD, Guerra M, et al. The effect of smoking on clinical and structural damage in patients with axial spondyloarthritis: a systematic literature review. Semin Arthritis Rheum. 2017; 46:569–83.

35. Molnar C, Scherer A, Baraliakos X, de Hooge M, Micheroli R, Exer P, et al. Rheumatologists of the Swiss Clinical Quality Management Program. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management Cohort. Ann Rheum Dis. 2018; 77:63–9.

36. Rosenbaum JT, Rosenzweig HL. Spondyloarthritis: the eyes have it: uveitis in patients with spondyloarthritis. Nat Rev Rheumatol. 2012; 8:249–50.
37. Rosenbaum JT, Asquith M. The microbiome and HLA-B27-associated acute anterior uveitis. Nat Rev Rheumatol. 2018; 14:704–13.

38. Kanski JJ, Bowling B. Kanski's clinical ophthalmology e-book: a systematic approach. 8th ed.Elsevier Health Sciences;2015.
Go to : 
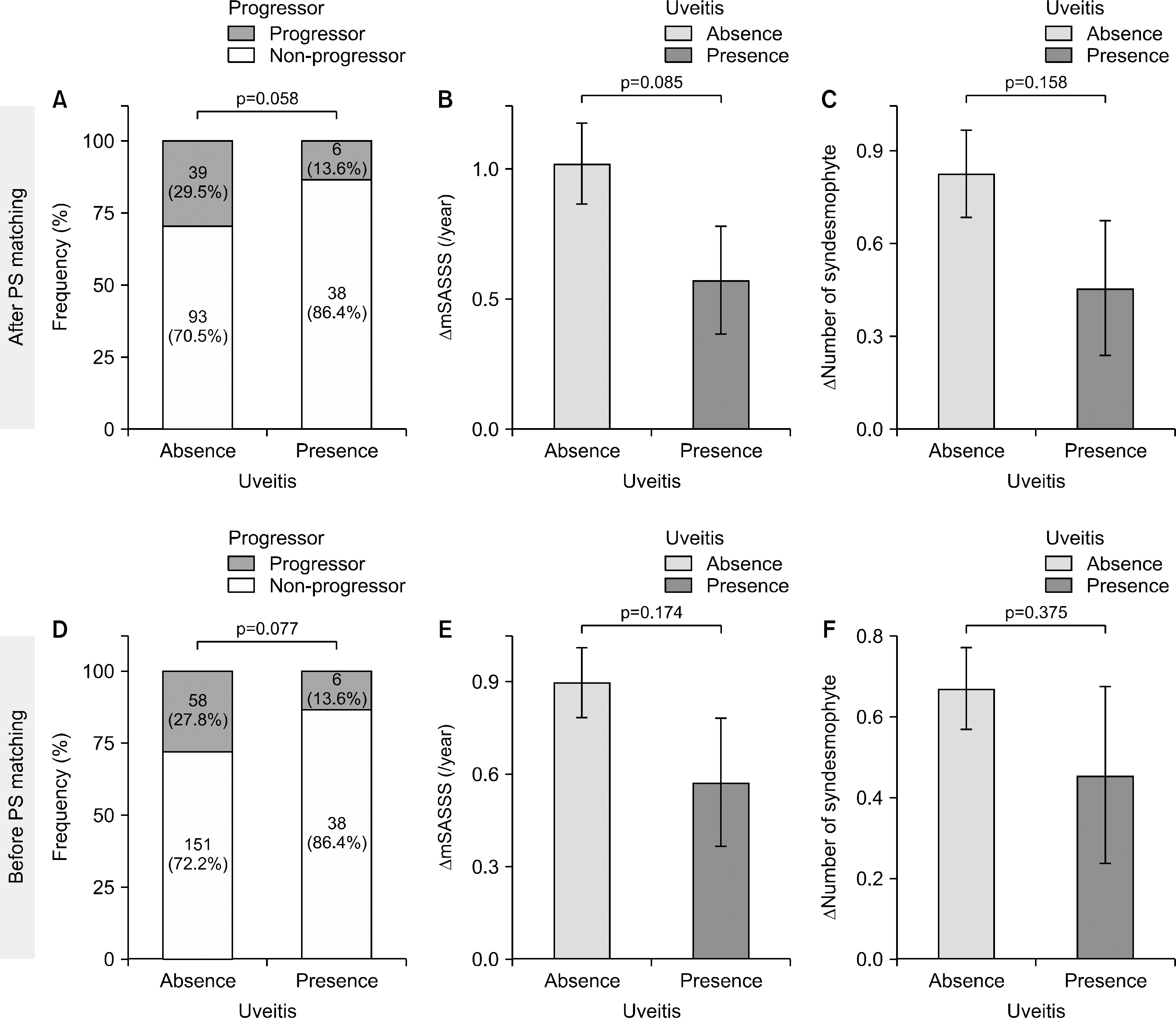 | Figure 1.Radiographic progression over 2 years in axSpA patients with AAU and those without AAU. Upper panel indicates the result after PS matching and lower panel before PS matching. (A, D) Proportion of progressors. Comparison by chi-square test. (B, E) Change in mSASSS. Comparison by t-test. (C, F) Change in number of syndesmophytes. Comparison by t-test. axSpA: axial spondyloarthritis, AAU: acute anterior uveitis, PS: propensity score, mSASSS: modified Stoke Ankylosing Spondylitis Spine Score. |
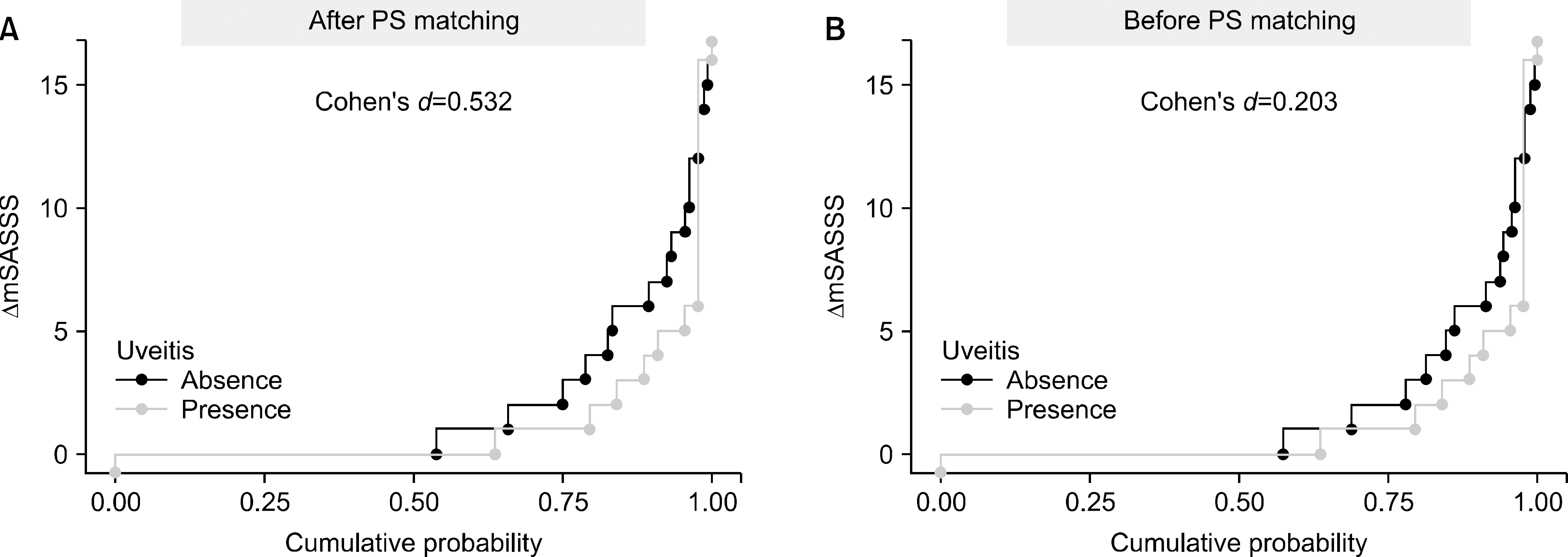 | Figure 2.Cumulative probability plot of 2-year progression in the modified Stoke Ankylosing Spine Score (mSASSS), illustrating the change (Δ) in mSASSS values from baseline of each individual radiographic interval to 2 years in patients with acute anterior uveitis (AAU) and those without AAU. Radiographic progression was defined as an increase in mSASSS ≥2 in 2 years. The effect size was computed by Cohen's d method. (A) After propensity score (PS) matching, (B) Before PS matching. |
Table 1.
Baseline characteristics of axSpA patients with and without AAU after propensity score matching
| Variable | Patients with AAU (n=44) | Patients without AAU | |||
|---|---|---|---|---|---|
| After PS matching (n=132) | p-value† | Before PS matching (n=209) | p-value‡ | ||
| Male | 34 (77.3) | 107 (81.1) | 0.744 | 153 (73.2) | 0.712 |
| Age at diagnosis (yr) | 34.2±12.6 | 33.3±12.4 | 0.672 | 32.7±12.1 | 0.190 |
| Disease duration (yr) | 4.48±4.96 | 4.61±5.46 | 0.878 | 3.88±5.18 | 0.227 |
| BMI (kg/m2) | 0.794 | ||||
| Underweight, <18.5 | 0 (0.0) | 1 (0.8) | 9 (4.3) | ||
| Normal, 18.5∼25 | 24 (54.5) | 80 (60.6) | 133 (63.6) | ||
| Overweight, 25∼30 | 16 (36.4) | 39 (29.5) | 52 (24.9) | ||
| Obese, <30 | 4 (9.1) | 12 (9.1) | 15 (7.2) | ||
| HLA-B27 | 42 (95.5) | 126 (95.5) | 1.000 | 175 (83.7) | 0.074 |
| Smoking | 0.861 | 0.578 | |||
| Non-smoker | 25 (56.8) | 71 (53.8) | 131 (62.7) | ||
| Smoker | 19 (43.2) | 61 (46.2) | 78 (37.3) | ||
| Peripheral arthritis | 8 (18.2) | 27 (20.5) | 0.913 | 77 (36.8) | 0.027 |
| Enthesitis | 0 (0.0) | 0 (0.0) | 1.000 | 13 (6.2) | 0.186 |
| Psoriasis | 1 (2.3) | 3 (2.3) | 1.000 | 3 (1.4) | 1.000 |
| Inflammatory bowel disease | 1 (2.3) | 1 (1.5) | 1.000 | 5 (2.4) | 1.000 |
| ESR (mm/h) | 30.2±21.4 | 31.5±25.1 | 0.768 | 36.1±27.6 | 0.122 |
| CRP (mg/dL) | 2.1±3.8 | 1.8±2.9 | 0.616 | 1.8±2.7 | 0.542 |
| ASDAS-CRP | 3.0±1.0 | 3.0±0.9 | 0.974 | 3.1±0.9 | 0.650 |
| Use of TNF inhibitor* | 25 (56.8) | 76 (57.6) | 1.000 | 114 (54.5) | 0.913 |
| Continuous use of NSAIDs | 17 (61.4) | 89 (67.4) | 0.582 | 140 (67.0) | 0.589 |
| mSASSS, units | 8.3±13.0 | 8.7±13.1 | 0.891 | 8.4±13.9 | 0.962 |
| Presence of syndesmophyte(s) | 19 (43.2) | 52 (39.4) | 0.790 | 82 (39.2) | 0.751 |
| Number of syndesmophyte(s) | 2.3±4.5 | 2.5±4.3 | 0.835 | 2.6±4.8 | 0.781 |
| Sacroiliac joint | 0.859/0.746 | 0.552/0.560 | |||
| Grade 0 (right/left) | 7 (15.9)/7 (15.9) | 16 (12.1)/18 (13.6) | 39 (18.7)/46 (22.0) | ||
| Grade 1 (right/left) | 6 (13.6)/6 (13.6) | 24 (18.2)/25 (18.9) | 45 (21.5)/38 (18.2) | ||
| Grade 2 (right/left) | 12 (27.3)/13 (29.3) | 42 (31.8)/35 (26.5) | 59 (28.2)/53 (25.4) | ||
| Grade 3 (right/left) | 8 (18.2)/7 (15.9) | 23 (17.4)/29 (22.0) | 32 (15.3)/38 (18.2) | ||
| Grade 4 (right/left) | 11 (25.0)/11 (25.0) | 27 (20.5)/25 (18.9) | 34 (16.3)/34 (16.3) | ||
| Hip involvement | 0.056/0.793 | 0.036/0.469 | |||
| Grade 0 (right/left) | 39 (88.6)/40 (90.9) | 121 (91.7)/123 (93.2) | 192 (91.9)/192 (91.9) | ||
| Grade 1 (right/left) | 2 (4.5)/1 (2.3) | 3 (2.3)/4 (3.0) | 7 (3.3)/9 (4.3) | ||
| Grade 2 (right/left) | 1 (2.3)/2 (4.5) | 8 (6.1)/3 (2.3) | 9 (4.3)/4 (1.9) | ||
| Grade 3 (right/left) | 0 (0.0)/0 (0.0) | 0 (0.0)/1 (0.8) | 1 (0.5)/3 (1.4) | ||
| Grade 4 (right/left) | 2 (4.5)/1 (2.3) | 0 (0.0)/1 (0.8) | 0 (0.0)/1 (0.5) | ||
| BMD (g/cm2) | |||||
| Lumbar spine | 1.050±0.136 | 1.060±0.168 | 0.932 | 1.060±0.157 | 0.802 |
| Femoral neck | 0.862±0.096 | 0.856±0.131 | 0.733 | 0.847±0.117 | 0.384 |
| Total hip | 0.942±0.104 | 0.924±0.119 | 0.337 | 0.907±0.107 | 0.047 |
| Z score | |||||
| Lumbar spine | −0.173±0.917 | −0.227±1.210 | 0.754 | −0.220±1.130 | 0.768 |
| Femoral neck | −0.118±0.900 | −0.264±0.852 | 0.350 | −0.386±0.804 | 0.073 |
| Total hip | −0.207±0.793 | −0.304±0.817 | 0.488 | −0.434±0.726 | 0.085 |
Values are presented as number (%) or mean±standard deviation. axSpA: axial spondyloarthritis, AAU: acute anterior uveitis, PS: propensity score, BMI: body mass index, ESR, erythrocyte sedimentation rate, CRP: c-reactive protein, ASDAS: Ankylosing Spondylitis Disease Activity Score, TNF: tumor necrosis factor, NSAIDs: non-steroidal anti-inflammatory drugs, mSASSS: modified Stoke Ankylosing Spondylitis Spine Score, BMD: bone mineral density.
Table 2.
Multivariable logistic regression analysis for radiographic progression in patients with axial spondyloarthritis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download