INTRODUCTION
Histiocytic sarcoma is a rare hematologic malignancy that shows histiocytic differentiation. Only a limited number of histiocytic sarcoma cases have been reported, and its exact etiology is unknown. This tumor mainly occurs at extranodal sites rather than in lymph nodes [
1], and according to the cases that have been reported so far, various extranodal sites, such as the skin, soft tissue, gastrointestinal tract, respiratory system, bone marrow, spleen, and central nervous system, can be affected [
1234].
A histologic examination is required for diagnosis, and the tumor itself can be confirmed to be a histiocytic sarcoma through the identification of its morphological features as well as the immunophenotype of its neoplastic cells; these tumors consist of large, noncohesive neoplastic cells surrounded by reactive inflammatory cells, with the neoplastic cells generally having an abundant eosinophilic cytoplasm. Furthermore, these cells are immunoreactive for cluster of differentiation (CD) 4, CD45, CD68, CD163, and lysozyme, and are negative for CD1a, CD3, CD20, CD21, and CD30 [
25].
There are no established guidelines for its treatment, and it is known to show an aggressive clinical course [
36]. As there has been very little published literature on cases of histiocytic sarcoma primarily involving the breast, we herein report a case of primary histiocytic sarcoma presenting as a breast mass in a 75-year-old patient.
CASE REPORT
A 75-year-old woman visited our breast clinic in July 2018 presenting with a palpable solitary mass in her right breast. She had a history of acute pyelonephritis, diabetes mellitus, essential hypertension, and rheumatoid arthritis; however, she had never been previously treated for malignant tumors. On physical examination, the patient reported no pain or tenderness in the mass.
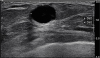
A subsequent breast ultrasonography conducted revealed the presence of a microlobulated hypoechoic mass of diameter 1.4 cm with increased vascularity in the upper inner quadrant of the right breast (
Figure 1), which was classified as Breast Imaging-Reporting and Data System (BI-RADS) category 4b, indicating a moderate suspicion for malignancy. Thereafter, an ultrasound-guided 14-gauge core needle biopsy was performed.
Figure 1
Breast ultrasonography shows a microlobulated hypoechoic mass in the right breast.
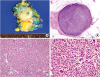
The histopathologic findings of the biopsy suggested that the tumor was a histiocytic neoplasm rather than a carcinoma as it was diffusely immunoreactive for CD68 and negative for pan-cytokeratin (AE1/AE3). Our pathology department then recommended a complete excision, and thus, a lumpectomy was performed in September 2018. On the cut section, a well-circumscribed, homogeneously yellowish-white, round solid mass was identified (
Figure 2A), and on subsequent microscopic examinations, it was found to be well-demarcated from the surrounding benign breast tissue and also showed a diffuse, noncohesive growth pattern (
Figures 2B and C). Furthermore, this solid mass mainly consisted of large pleomorphic neoplastic cells, some multinucleated neoplastic giant cells, and inflammatory components (
Figure 2D), with the tumor cells composed of large, oval/irregularly folded nuclei with vesicular chromatin and an abundant eosinophilic cytoplasm (
Figure 2D). Here, mitosis was observed at a rate of approximately 5 mitoses/10 high-power field (original magnification ×400), and the Ki-67 labeling index was 10%. No necrosis or hemorrhage was observed.
Figure 2
Histopathologic features of the histiocytic sarcoma. (A) The round solid mass is approximately 1.5 cm × 1.2 cm in size and is surrounded by breast tissue. (B) In the scanning view, the tumor is well-demarcated and shows high cellularity. (C) The tumor cells show a diffuse, noncohesive growth pattern and various admixed inflammatory components are also identified (hematoxylin-eosin stain, original magnification ×100). (D) Large atypical cells with an abundant eosinophilic cytoplasm as well as multinucleated giant cells are frequently observed. Mitosis is also easily observed. (hematoxylin-eosin stain, original magnification ×400).
On immunohistochemistry (IHC), these tumor cells showed strong and diffuse immunoreactivity for CD68, CD163, and HLA-DR, and also showed a weak to moderate diffuse immunoreactivity for CD45 and CD4, while the IHC results for CD21 and CD1a were negative (
Figures 3 and
Table 1). We also performed IHC for pan-cytokeratin, CD3, CD8, CD20, CD79a, CD138, CD30, epithelial membrane antigen, Melan A, CD34, CD117, and Myeloperoxidase in order to distinguish this tumor from carcinomas, other lymphomas, malignant melanomas, and myeloid sarcoma; the results for these were all negative (
Supplementary Figure 1). Based on the observed morphology and immunophenotype, the tumor was ultimately diagnosed as a histiocytic sarcoma.
Figure 3
Results of the immunohistochemistry (original magnification ×400). The tumor cells show strong and diffuse immunoreactivity for CD68 (A) and CD163 (B), and are negative for CD21 (C) and CD1a (D).
CD = cluster of differentiation.
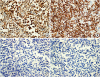
Table 1
The immunohistochemical profile of the patient, histiocytic sarcoma, Langerhans cell sarcoma, and dendritic cell neoplasms
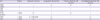
|
IHC |
Patient |
Histiocytic sarcoma |
Langerhans cell sarcoma |
Follicular dendritic cell sarcoma |
Interdigitating dendritic cell sarcoma |
|
CD68 |
+ |
+ |
+/− |
+/− |
+/− |
|
CD163 |
+ |
+ |
− |
− |
− |
|
CD21 |
− |
− |
− |
+ |
− |
|
CD1a |
− |
− |
+ |
− |
− |
|
S-100 |
+, weak |
+/− |
+ |
+/− |
+ |
|
HLA-DR |
+ |
+ |
+ |
+ |
+ |
|
CD45 |
+, weak |
+/−, frequently |
+/− |
− |
+/− |
|
CD4 |
+, weak |
+/− |
+/− |
|
+/− |
Thereafter, due to the possibilities that the lesion may have metastasized elsewhere or the lesion itself may have metastasized from other sites, a whole body positron-emission tomography/computed tomography using deoxy-2-[18F]fluoro-D-glucose was performed, and the images showed no other masses or lesions. Thus, we considered this mass to be a primary histiocytic sarcoma of the breast.
After surgical excision, chemotherapy was not performed as no systemic involvement was observed. The patient was followed up for eight months, and no evidence of tumor recurrence or metastasis was noted.
This study was approved by the Institutional Review Board of Hanyang University Hospital (HYUH 2018-11-027-001), and the requirement for informed consent was waived.
DISCUSSION
Histiocytic sarcoma is known to occur in various organs; however, its association with the breast has rarely been reported. There have been a few reported case studies of histiocytic sarcoma invasions into the breast; however, the reported primary sites of the tumor in these case studies were the soft tissue of axilla and the subcutaneous breast tissue [
27]. To the best of our knowledge, this case study is the first report of primary histiocytic sarcoma of the breast parenchyma.
Patients with histiocytic sarcoma may present with various systemic symptoms, including fever, weight loss, and generalized weakness [
8]. However, extranodal histiocytic sarcomas are often observed to be painless solitary masses, and thus, diagnosis may be difficult to conduct [
9]. Moreover, the imaging findings are generally nonspecific and a histologic examination is also essential for the diagnosis. On histologic examination, a diffuse proliferation of large atypical cells with an abundant eosinophilic cytoplasm is characteristically observed, and various degrees of inflammatory cells may be observed as well. A definitive diagnosis of histiocytic sarcoma is difficult to achieve, but various other neoplastic diseases should additionally be ruled out. The differential diagnoses here include large cell non-Hodgkin lymphomas (such as diffuse large B-cell lymphoma and anaplastic large cell lymphoma), poorly differentiated carcinomas, malignant melanomas, myeloid sarcoma, and other histiocytic and dendritic cell neoplasms [
6910]. Here, IHC is also useful to differentiate the histiocytic sarcoma from the other tumors, and these large atypical cells show strong and diffuse immunoreactivity for CD163, CD68, and lysozyme. CD163 is more specific than CD68, and strong immunoreactivity for this antigen is suggestive of histiocytic differentiation [
11]. The detailed IHC results of our case, as well as the IHC profiles of histiocytic and dendritic cell neoplasms are presented with this report (
Table 1). Until recently, the genetic profile of histiocytic sarcoma was not clearly defined; some studies have reported
BRAF mutations, including V600E, which suggests that
BRAF-target therapy could be promising [
121314].
BRAF V600E IHC was performed in our case, and the result was negative. Additionally, there are no established guidelines for the treatment of histiocytic sarcoma. Several literatures reported that extranodal histiocytic sarcomas with localized involvement were often treated with surgical treatment. In case of an advanced stage tumor, chemotherapy and radiotherapy may be considered, but the number of cases was so small that it was difficult to predict the effect of this treatment on prognosis [
615]. For instance, primary histiocytic sarcoma of the central nervous system is known to have a poor prognosis [
4], that the tumor of systemic involvement shows a more aggressive clinical course, and that the localized masses show a better prognosis [
14]. However, the prognosis is unclear for tumors in uncommon sites such as the one in our present case.
Our patient did not experience any tumor recurrence or metastasis; however, the follow-up period was relatively short. Although localized and small primary tumors have a favorable prognosis, histiocytic sarcoma is generally known to be an aggressive tumor. Therefore, a close follow-up is mandatory in such cases.
In conclusion, we reported a rare case of primary histiocytic sarcoma that occurred in the breast parenchyma. Although the diagnosis of histiocytic sarcoma is difficult to achieve, pathologists should consider pan-cytokeratin IHC to exclude tumors of epithelial origin even if they are in breast parenchyma when diagnosing tumors showing cells with pleomorphic morphology. Further research is required to define the molecular alterations and prognosis of this tumor.







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download