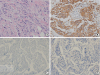
A 49-year-old female patient presented with left periductal mastitis with nipple retraction in April 2018. Her chief complaint was left breast swelling with a febrile sensation. The patient had a previous history of invasive ductal carcinoma of the left breast in May 2012, which was managed with breast-conserving surgery and sentinel lymph node biopsy. The breast carcinoma was estrogen (prediluted, SP1; Ventana Medical Systems, Inc., Tucson, USA) and progesterone (prediluted, 1E2; Ventana Medical Systems, Inc.) receptor-negative, and the HER2 (prediluted, 4B5; Ventana Medical Systems, Inc.) score was 3+. The histopathologic result in the first surgery indicated HER2-subtype invasive ductal carcinoma. The tumor cells invaded the stroma in the form of linear strands or cords of various sizes. The nuclei of the neoplastic cells moderately varied in size; they had limited tubule formation capacity and a moderate mitotic count. HER2 immunohistochemistry showed 3+ immunoreactivity within the circumferential membrane of the tumor cells (
Figure 1A and B). The basal markers cytokeratin (CK) 5/6 and epidermal growth factor receptor (EGFR) tested negative. Following the diagnosis of the relapsed mass in 2018, the basal markers that were investigated in the recurrent mass were re-examined in the first surgical specimen. Even after repeated immunostaining for CK 5/6 (1:100; B5/16 B4; DAKO, Kyoto, Japan) and EGFR (1:50; SP84; Cell Marque, Rocklin, USA), negative results were obtained (
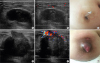
Figure 1C and D). The tumor–node–metastasis stage was stage IIA, T1cN1M0. After the first surgery, she received 4 cycles of doxorubicin and cyclophosphamide treatment, followed by 4 cycles of docetaxel. After 6 months of chemotherapy, radiation therapy was also completed, and 18 cycles of trastuzumab treatment were administered for 1 year. Thereafter, she was regularly followed up for the next 4 years. However, she skipped the scheduled examination in November 2017. After that, she visited the clinic with left periductal mastitis in April 2018. Breast ultrasound was performed, revealing a complex solid and cystic mass (approximately 3.5 × 1.9 cm) in the left upper central breast. The skin overlying her left breast showed diffuse thickening (
Figure 2A and B). The cystic lesion was aspirated, and the aspiration fluid was turbid and yellowish. The aspiration fluid was cultured, and no bacteria were found. She was treated on the basis of suspected mastitis due to the abscess in the left breast. In May 2018, the abscess was incised, drained, and cultured again; however, no cultured bacteria were found (
Figure 2C). After the initial incision and drainage, the tumor had enlarged. The size of the complex mass increased to approximately 5.5 × 3.8 cm (
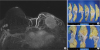
Figure 2D and E). A core-needle biopsy of the left breast lesion revealed squamous cell carcinoma. She opted to undergo left breast mastectomy, and as a preoperative evaluation, breast magnetic resonance imaging was performed, which showed a left breast mass abutting the pectoralis muscle with enlarged right axillary lymph nodes (
Figure 3A). Positron emission tomography-computed tomography was performed to evaluate other metastatic lesions. Except for that in the right axillary lymph nodes, metastasis was not detected. In July 2018, her second surgery was performed because of relapsed left breast cancer. She underwent a left simple mastectomy and right axillary lymph node biopsy (
Figure 3B and C). The histopathological result of the second surgery was metaplastic squamous cell carcinoma of basal-HER2 subtype. The course of treatment was decided by a multidisciplinary team, with inputs from various experts in the departments of surgery, oncology, pathology, and radiology. Her invasive ductal carcinoma had been locally controlled since 2012. Therefore, the best course of action was decided to be the repeated anthracycline and taxane chemotherapy, followed by trastuzumab, postmastectomy radiation therapy, tamoxifen, and goserelin.
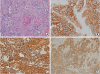
The tumor was completely resected after the surgical treatment. Left axillary lymph node dissection was not performed because no enlarged or suspected malignant lymph nodes were detected during the second surgery. A frozen biopsy of the right axillary lymph nodes yielded negative results for malignancy. Therefore, right axillary lymph node dissection was not performed. No recurrence was observed, and chemotherapy is currently ongoing. The relapsed cancer in 2018 was pure squamous cell carcinoma and showed positive results for the breast cancer marker GATA binding protein 3. This recurring mass consisted of large polygonal malignant cells containing intercellular bridges and abundant eosinophilic cytoplasm with nuclear atypia and focal pycnotic keratinization found in squamous cell carcinoma (
Figure 4A). The immunophenotype of this recurred mass was determined to be the basal-HER2 subtype. The Allred scores for estrogen and progesterone receptors were 2 and 6, respectively, and the HER2 score was 3+. The recurrent tumor cells showed strong expression of CK 5/6 in their cytoplasm and cell membrane and diffuse moderate membranous staining of EGFR (
Figure 4B-D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download