INTRODUCTION
METHODS
Reagents
Cell lines
Cell-based STAT92E reporter assay
Cell viability assay
Western blot analysis
Immunoprecipitation assay
Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR)
Immunofluorescence staining
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Statistics
RESULTS
Identification of tubulosine as an inhibitor of STAT92E activity in Drosophila cells
 | Figure 1Tubulosine inhibits Drosophila STAT92E activity. (A) The chemical structure of tubulosine. (B) Tubulosine inhibits Upd-induced STAT92E transcriptional activity. S2-NP-STAT92E cells were co-cultured with Upd-producing S2-NP cells for 24 hours in the presence of the vehicle (0.1% dimethyl sulfoxide) alone or the indicated concentrations of tubulosine. STAT92E reporter activity was normalized using the ratio of firefly luciferase to Renilla luciferase activity. Reporter activity without Upd stimulation was set to 1. (C) Tubulosine inhibits Upd-induced STAT92E tyrosine phosphorylation. S2-NP cells were incubated with various concentrations of tubulosine for 24 hours in the presence or absence of Upd. Immunoprecipitation and Western blot analyses were performed to determine the levels of pY-STAT92E and STAT92E. (D) Cells were prepared as described in (B), and cell viability was determined using EZ-CyTox Enhanced Cell Viability Assay reagent. Cell viability was represented as a control % compared to the vehicle-treated group. Results are represented as means ± standard deviation of 3 independent experiments (n = 3).STAT92E = signal transducer and activator of transcription protein at 92E; Upd = Unpaired.
*p < 0.005 compared to the vehicle-treated group; †p < 0.05 and ‡p < 0.005 compared to the Upd-induced group.
|
Tubulosine inhibits IL-6-induced JAK2/STAT3 signaling
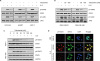 | Figure 2Tubulosine inhibits IL-6-induced JAK2/STAT3 signaling in breast cancer cells. (A-C) Cells were incubated for 6 hours in the presence of the vehicle (0.1% dimethyl sulfoxide) alone or tubulosine (100 nM or the indicated concentrations) and then stimulated with IL-6 (20 ng/mL) for 10 minutes. Protein samples were prepared from whole-cell lysates and Western blot analyses were performed. GAPDH was used as a loading control. (D) Cells were prepared as described in Figure 2A-C, followed by IL-6 stimulation for 30 minutes and then performed immunofluorescence staining. Nuclei were counterstained with DAPI.IL = interleukin; JAK2 = Janus kinase 2; STAT3 = signal transducer and activator of transcription 3; PBS = phosphate-buffered saline; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; DAPI = 4′,6-diamidino-2-phenylindole.
|
Tubulosine blocks the binding of IL-6Rα and gp130
 | Figure 3Tubulosine blocks the binding of IL-6Rα and gp130. (A) MCF-7 cells were incubated with tubulosine (100 nM) for the indicated periods and then stimulated with IL-6 (20 ng/mL) for 10 minutes. Protein samples were prepared from whole-cell lysates and Western blot analyses were performed. GAPDH was used as a loading control. (B and C) MCF-7 cells were incubated for 3 hours with the vehicle (0.1% dimethyl sulfoxide) alone or tubulosine and then stimulated with IL-6 (20 ng/mL) for 10 minutes. Immunoprecipitation was performed for whole-cell lysates using an anti-IL-6Rα (B) or anti-gp130 antibody (C) followed by Western blot analyses.STAT3 = signal transducer and activator of transcription 3; IL = interleukin; IL-6Rα = interleukin-6 receptor α; GAPDH = glyceraldehyde 3-phosphate dehydrogenase.
|
Tubulosine induces apoptotic cell death
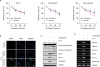 | Figure 4Tubulosine induces apoptotic cell death. (A) Cells were incubated with tubulosine (100 nM) or AG-490 (150 µM) for the indicated periods in the presence of IL-6 (20 ng/mL). Cell viability was determined using EZ-CyTox Enhanced Cell Viability Assay reagent and represented as a control % compared to the vehicle-treated group. Results are represented as means ± SD of three independent experiments (n = 3). (B and C) MCF-7 cells were incubated for 48 hours with the vehicle (0.1% DMSO) alone or tubulosine (100 nM) in the presence or absence of IL-6 (20 ng/mL). A TUNEL assay (B) and Western blot analyses (C) were performed. (D) MCF-7 cells were incubated for 3 hours with the vehicle (0.1% DMSO) alone or tubulosine (100 nM), and further incubated for 12 hours in the presence or absence of IL-6 (20 ng/mL). Semi-quantitative RT-polymerase chain reaction was performed using target-specific primers.TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling; DAPI = 4′,6-diamidino-2-phenylindole; DMSO = dimethyl sulfoxide; IL = interleukin; Bcl = B-cell lymphoma; PARP = poly (ADP-ribose) polymerase; MMP = matrix metalloproteinase.
*p < 0.05 and †p < 0.005 compared to the vehicle-treated group.
|
DISCUSSION
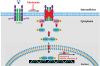 | Figure 5Schematic diagram representing the proposed mechanism of action of tubulosine. Tubulosine inhibits IL-6-induced JAK2/STAT3 signaling by blocking IL-6Rα and gp130 binding.IL = interleukin; IL-6Rα = interleukin-6 receptor α; JAK2 = Janus kinase 2; STAT3 = signal transducer and activator of transcription 3; gp130 = glycoprotein 130.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download