Abstract
Purpose
We evaluated the motion-induced dosimetric effects on the field-in-field (FIF) technique for whole-breast irradiation (WBI) using actual patient organ motion data obtained from cine electronic portal imaging device (cine EPID) images during treatment.
Materials and Methods
Ten breast cancer patients who received WBI after breast-conserving surgery were selected. The static FIF (SFIF) plan involved the application of two parallel opposing tangential and boost FIFs. To obtain the amplitude of the internal organ motion during treatment, cine EPID images were acquired five times for each patient. The outside contour of the breast (OCB) and chest wall (CW) contour were tracked using in-house motion analysis software. Intrafractional organ motion was analyzed. The dynamic FIF (DFIF) reflecting intrafractional organ motion incorporated into the SFIF plan was calculated and compared with the SFIF in terms of the dose homogeneity index (DHI90/10) for the target and V20 for the ipsilateral lung.
Results
The average motion amplitudes along the X and Y directions were 1.84±1.09 mm and 0.69±0.50 mm for OCB and 1.88±1.07 mm and 1.66±1.49 mm for CW, respectively. The maximum motion amplitudes along the X and Y directions were 5.53 and 2.08 mm for OCB and 5.22 and 6.79 mm for CW, respectively. Significant differences in DHI90/10 values were observed between SFIF and DFIF (0.94 vs 0.95, P<0.05) in statistical analysis. The average V20 for the lung in the DFIF was slightly higher than that of the SFIF in statistical analysis (19.21 vs 19.00, P<0.05).
The whole-breast irradiation (WBI) technique has attracted considerable interest because breast cancer is one of the most prevalent cancers worldwide; the number of breast cancer patients has rapidly increased in recent times.1) WBI has played an important role in minimizing the risk of ipsilateral recurrence after breast-conserving surgery.2345) WBI has traditionally been performed with tangential irradiation (TI), which involves the application of parallel opposing half-beam wedge fields. Conventional TI is very simple and convenient, but dose inhomogeneity in the target volume resulting from tissue heterogeneity in the irradiated volume and the difference in beam path lengths is an unavoidable demerit. Overdosage can lead to unwanted cosmetic outcomes and side effects.67) In addition, TI is not suitable to modify the beam intensity at specific regions, and modification is generally used for reducing the radiation dose to normal organs.
With this background, Oliver et al. employed intensity-modulated radiation therapy (IMRT) for WBI to obtain a homogeneous dose distribution in the target volume and reduce dosage to normal healthy tissue.891011) However, while IMRT is advantageous from the dosimetric point of view, the technique suffers from the demerits of long planning and treatment time, large number of MUs, and relative complexity of treatment compared with TI.12) Mihai et al.13) introduced the forward intensity-modulated radiation therapy (FIMRT) technique for WBI. FIMRT essentially involves the application of parallel opposing open fields to cover the entire target volume and sub-boost fields, which are shaped by a multi-leaf collimator to compensate for dose inhomogeneity within the target volume and reduce dosage to normal organs. In comparison with IMRT, FIMRT offers simplicity of treatment technique, good dose homogeneity in the target volume, a small number of MUs, and reduced delivery time. Prabhakar et al. examined different types of FIMRT and proposed the field-in-field (FIF) technique, which offers all the advantages of the IMRT, FIMRT, and TI techniques.1415)
The target volume for WBI includes the tumor bed and the whole-breast tissue on the CW. The target volume is expected to be subject to geometrical uncertainty because of the influence of respiratory organ motion.16) The IMRT, FIMRT, and FIF techniques are disadvantageous when considering organ motion in comparison with simple TI because their beams consist of many sub-fields of different radiation intensities to obtain the desirable dose distribution within the treatment volume. Jain et al.17) have reported that the organ motion effect is not significant from the dosimetric point of view when FIMRT is used for WBI. Song et al.18) conducted similar research on the FIF technique and reported significant dose variation because of respiratory organ motion by applying speculative motion values (1, 2, and 3 cm) instead of actual patient data. However, to the best of our knowledge, no study has addressed the dosimetric effects of target motion based on actual patient motion data with patient-specific treatment beam conditions. Thus, the dosimetric effects of respiratory organ motion need to be evaluated by applying actual patient data for the breast FIF technique to ensure better outcomes and safer treatment. We evaluated the motion-induced dosimetric effects on the breast FIF technique using actual patient organ motion data obtained from cine electronic portal imaging device (cine EPID) images during treatment.
Ten breast cancer patients were randomly selected (five right-breast and five left-breast cancer patients) for WBI in this study after breast-conserving surgery (details are listed in Table 1). A planning computed tomography (CT) was performed for each patient in the supine position with the arm up on the breast board (Medtech, USA). All CT images were transferred to the treatment planning system (TPS, Pinnacle3, Philips Medical Systems, Milpitas, CA, USA). We delineated the clinical target volume (CTV) including the tumor bed and breast tissue and organs at risk (OARs) including the ipsilateral lung and heart. The FIF technique, one of the FIMRT techniques, was applied in combination with TI (Fig. 1a) and sub-boost fields (Fig. 1b, c) to improve dose distribution, as described below. First, TI beams were generated via the application of two open parallel opposing half-beams (6-MV photons, CL600, Varian, USA). The initial dose distribution was calculated with equal beam weights. Next, an isodose cloud was displayed on the digital reconstruction radiograph (DRR). Our plan criterion for WBI was that the dose received by the CTV should lie in the range of 95% to 107% of the prescribed dose. Therefore, an isodose cloud outside this range was acquired, and the dose was compensated manually using a multileaf collimator (MLC) (Fig. 1). In other words, when the CTV received a dose less than 95% of the prescribed dose, a sub-field was added to compensate for the dose, and when the CTV dose exceeded 107%, shielding was applied via the MLC. The total number of sub-fields was limited to less than two for the treatment plan of each patient. Furthermore, we ensured that the total beam weight did not exceed 10% of the entire beam weight. A total of 50 Gy with 25 fractions was prescribed for the CTV.
Cine EPID images were used to measure the intrafractional motion of the breast. Cine EPID imaging is used to obtain continuous images with the beam used in the actual treatment with an EPID (aS500, Varian, USA) during treatment, and the technique is suitable for real-time verification of organ motion.19) Cine EPID images were obtained at a rate of 3.3 frames/s once a week per patient five times during the entire treatment schedule.
We developed an in-house motion analysis software package to extract the motion of the breast from the acquired sequential cine EPID images (Matlab, MathWorks, USA) (Fig. 2). The outside contour of the breast (OCB) and the chest wall (CW) at the central axis of the beam were set as the region of interest (ROI), and the motion of the ROI in the sequential cine EPID was tracked by applying a pattern matching algorithm. Variations along the X and Y directions were measured with respect to the vertical axis of the tangential beam direction, and based on these values, the maximum amplitude, mean amplitude, and the standard deviation were calculated.
To analyze the dosimetric effects of the intrafractional variation due to respiration with regard to the FIF technique, a dynamic FIF (DFIF) plan was generated; this plan reflects the motion measured by cine EPID onto the static FIF (SFIF) plan, which does not reflect organ motion. In other words, DFIF dose distribution was recalculated by adjusting the position of the sub-fields based on each patient's specific movement values, as measured by cine EPID (the maximum amplitude of motion for the OBC and CW). We calculated the cumulative dose-volume histogram (DVH) for PTV and OARs from both SFIF and DFIF for all cases. Furthermore, for quantitative analysis of motion-induced dosimetric effects, the dose homogeneity index (DHI) for CTV and the percentage volume receiving over 20 Gy (V20) for the ipsilateral lung were calculated and compared for both techniques.1820)
DHI indicates dose uniformity within the CTV, and it is defined as the ratio of the treatment volume receiving 10% of the prescription dose (D10) to that receiving 90% (D90). A DHI value of 1 is an ideal value that indicates uniform dose distribution within the CTV. To assess whether there is significant difference between the SFIF and DFIF, a Wilcoxon signed-rank test (version 9.4, SAS Institute, Cary, NC) was performed. A P-value of <0.05 was considered statistically significant.
We analyzed all 50 sets of cine EPID images obtained for the 10 patients and determined that the mean maximum motion amplitudes in all patients were 1.84±1.09 mm and 0.69±0.50 mm along the X and Y directions in the OCB, respectively, and 1.88±1.07 mm and 1.66±1.49 mm in the CW, respectively (Table 2). When the motion of the CW was slightly greater than that of the OCB, the error along the X direction was greater than that along the Y direction. The statistics of each patient's motion showed a considerable difference. In patient #2, the CW motion was 6.79 mm along the Y direction, whereas it was 0.66 mm in patient #8, which corresponded to a large difference of about 6 mm. Moreover, patients with large OCB motion generally exhibited large CW motion as well. Among all the patients, the maximum OCB motion amplitudes were 5.53 and 2.08 mm and the maximum CW motion amplitudes were 5.22 and 6.79 mm along the X and Y directions, respectively.
We used a total of 20 sub-fields in this study to perform the FIF plan, which corresponds to 2 sub-fields per patient on an average. In most patients, prominent improvement in the dose distribution, reduction in dose inhomogeneity within the target (white arrow in Fig. 3), and reduction in the lung dose (red arrow in Fig. 3b) were observed because of the addition of the sub-fields.
When the DFIF (Fig. 4b) was calculated by applying the maximum intrafractional movement measured from each patient to the SFIF (Fig. 4a), variations were observed not only in the dose distribution within the target but also in the lung dose (red arrow). Such a dose change was evident in the DVH. In one extreme case, the ipsilateral lung dose and target dose slightly increased (Fig. 5).
The mean DHI90/10 value for the target with SFIF for all 10 patients was 0.94±0.01 (Table 3). The mean DHI90/10 with DFIF as generated for maximum movement along the +X direction was 0.94±0.01, and it was 0.95±0.01 along the −X direction. The mean DHI90/10 with DFIF as calculated for maximum movement along the +X and +Y directions simultaneously was 0.94±0.01 (P>0.05), and it was 0.95±0.01 (P<0.05) for simultaneous maximum movement along the −X and −Y directions. However, the differences between all these values did not exceed 1%.
The V20 value of the ipsilateral lung with SFIF was 19.00±7.16. The V20 values of the ipsilateral lung with DFIF were 18.89±7.16 and 19.21±7.17 for the maximum movement along the +X and −X directions, respectively. Furthermore, the V20 value was 18.90±7.15 for simultaneous movement along the +X and +Y directions, and it was 19.20±7.18 for simultaneous movement along the −X and −Y directions (P<0.05). However, the differences between all these values did not exceed 1%.
Many researchers have reported organ movement during WBI. Saliou et al.21) obtained portal films of TI during treatment, analyzed the central lung distance (CLD), and reported movements of 0.8~10 mm along the anterior-posterior direction. Smith et al. obtained a total of 1,709 electronic portal images, analyzed the CLD, and observed a maximum CLD variation of 2.5 mm. This result indicates that little fluctuation was caused by breathing during treatment.22) Baroni et al. attached markers on breast skin and monitored their motion in real time using an opto-electronic system. Their analysis on motion caused by breathing during treatment showed that the median was consistent at 2–3 mm and exhibited the highest value along the anterior-posterior direction.23) Richter et al. analyzed the motion of the chest using four-dimensional CT (4DCT) in 10 patients, and the maximum motion amplitude was less than 4 mm in all patients. Furthermore, they compared the motion amplitude obtained via cine EPID images to that obtained via 4DCT. The results were similar in both cases.24) In our study, we obtained an average motion of less than 2 mm, which was consistent with the results of other studies. However, the maximum amplitude of motion was significantly different from patient to patient. In addition, considerable motion was observed in some patients.
One of the demerits of treatment techniques that use sub-fields, such as IMRT and FIF, is that they are more sensitive to dosimetric error by intrafractional motion than TI. Although the FIF uses only a few sub-fields, their influence cannot be completely excluded. The interplay effect, which is the mismatch between the target motion and delivery timing of these multiple sub-fields, is strongly associated with not only organ motion but also patient-specific conditions including treatment techniques and anatomical geometry.2526) The changes in dose distribution because of respiratory motion have been investigated in many studies. However, the results are inconsistent because these studies employed different types of information for dosimetric evaluation instead of actual patient data.16182728) To overcome this issue, we evaluated the motion-induced dosimetric effects on the breast FIF technique by utilizing actual patient planning and organ motion data obtained from cine EPID images during treatment. Because of respiration, the DHI of the target volume exhibited a maximum difference of 1.06%, and the dosage of the ipsilateral lung exhibited a partial increase when the sub-field was applied along the direction of the chest wall close to the lung. However, these differences were not clinically significant.
We evaluated the effects of motion-induced dosimetric error by applying actual patient motion data that were obtained via cine EPID images for FIF WBI. Dose variation with regard to the FIF technique due to respiratory organ motion was observed in both the target and lung volumes as per statistical analysis; however, the difference in values was clinically acceptable. Our findings indicate that FIF can form a safe and effective treatment method for the radiation treatment of breasts as it can improve dose distribution within the target volume and reduce normal organ dose. We believe that the establishment of motion criteria and regular monitoring using cine EPID images can be effective in reducing motion-induced dosimetric errors.
Figures and Tables
Fig. 1
Design of field-in-field (FIF) treatment plan. The FIF plan involves the application of (a) two tangential beams and (b, c) sub-boost fields to compensate for the dose inhomogeneity in the target and reduce the lung dose.

Fig. 2
(a) In-house motion analysis software and (b) the result of motion analysis for whole-breast irradiation. Solid-lines (breast skin contour) and dotted (chest wall contour) boxes represent surrogates for organ motion tracking based on a pattern matching algorithm.
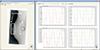
Fig. 3
Comparison of dose distribution (sagittal view) between the application of (a) a conventional tangential field and (b) a conventional field-in-field (FIF) boost technique. Regions subject to high dosage with the conventional field exhibited a significant reduction with the FIF technique (white arrow) along with significant reduction in the lung dose also (red arrow).
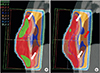
Fig. 4
Comparison of dose distribution between (a) static FIF (SFIF) and (b) dynamic FIF (DFIF). A partial dose increase in the lung (red arrow) and dose decrease in the target (white arrow) were observed for the DFIF plan because of organ motion.
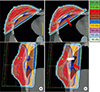
Fig. 5
An extreme example of dose-volume histogram (DVH) for target volume and lung. The ipsilateral lung dose and target dose increased slightly in dynamic FIF (DFIF, dotted line, applied maximum of the organ motion along -X direction) compared to static FIF (SFIF).

Table 2
Statistics of intrafractional movement from cine-EPID images obtained using in-house motion analysis software for whole-breast irradiation
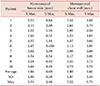
Table 3
Dose variation due to organ motion

DHI90/10, dose homogeneity index; SFIF, static field-in-field; DFIF+XYmax, dynamic field-in-field applied max. organ motion amplitude along +X and +Y directions; DFIF−XYmax, dynamic field-infield applied max. organ motion amplitude along −X and −Y directions.
A significant difference in DHI90/10 for CTV and V20 for lung was observed between the SFIF and DFIF (P<0.05) in statistical analysis, but the difference in values was clinically acceptable.
References
1. International Agency for Research on Cancer (IARC) and World Health Organization (WHO). GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide 2012. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
2. Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson CK, Andersen KW, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr. 1992; 19–25.
3. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.

4. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–1232.

5. Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995; 333:1456–1461.

6. Taylor ME, Perez CA, Halverson KJ, Kuske RR, Philpott GW, Garcia DM, et al. Factors influencing cosmetic results after conservation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 1995; 31:753–764.

7. Park W, Huh SJ, Yang JH, Nam SJ, Kim JH, Choi JY, et al. The implication of hot spots on bone scans within the irradiated field of breast cancer patients treated with mastectomy followed by radiotherapy. Ann Nucl Med. 2008; 22:685–691.

8. Oliver M, Chen J, Wong E, Van Dyk J, Perera F. A treatment planning study comparing whole breast radiation therapy against conformal, IMRT and tomotherapy for accelerated partial breast irradiation. Radiother Oncol. 2007; 82:317–323.

9. Prabhakar R, Haresh KP, Julka PK, Ganesh T, Rath GK, Joshi RC, et al. A study on contralateral breast surface dose for various tangential field techniques and the impact of set-up error on this dose. Australas Phys Eng Sci Med. 2007; 30:42–45.

10. Harsolia A, Kestin L, Grills I, Wallace M, Jolly S, Jones C, et al. Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys. 2007; 68:1375–1380.

11. Kestin LL, Sharpe MB, Frazier RC, Vicini FA, Yan D, Matter RC, et al. Intensity modulation to improve dose uniformity with tangential breast radiotherapy: initial clinical experience. Int J Radiat Oncol Biol Phys. 2000; 48:1559–1568.

12. Haciislamoglu E, Colak F, Canyilmaz E, Dirican B, Gurdalli S, Yilmaz AH, et al. Dosimetric comparison of left-sided whole-breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and volumetric arc therapy. Radiother Oncol. 2011; 100:241–246.

13. Mihai A, Rakovitch E, Sixel K, Woo T, Cardoso M, Bell C, et al. Inverse vs. forward breast IMRT planning. Med Dosim. 2005; 30:149–154.

14. Onal C, Sonmez A, Arslan G, Oymak E, Kotek A, Efe E, et al. Dosimetric comparison of the field-in-field technique and tangential wedged beams for breast irradiation. Jpn J Radiol. 2012; 30:218–226.

15. Prabhakar R, Julka PK, Rath GK. Can field-in-field technique replace wedge filter in radiotherapy treatment planning: a comparative analysis in various treatment sites. Australas Phys Eng Sci Med. 2008; 31:317–324.

16. Michalski A, Atyeo J, Cox J, Rinks M. Inter- and intra-fraction motion during radiation therapy to the whole breast in the supine position: a systematic review. J Med Imaging Radiat Oncol. 2012; 56:499–509.

17. Jain P, Marchant T, Green M, Watkins G, Davies J, McCarthy C, et al. Inter-fraction motion and dosimetric consequences during breast intensity-modulated radiotherapy (IMRT). Radiother Oncol. 2009; 90:93–98.

18. Song T, Suh CO, Lee I, Jeong K, Keum K, Lee CG, et al. The effect of respiratory motion on forward intensity modulated radiotherapy for breast cancer. Technol Cancer Res Treat. 2008; 7:207–215.

19. Mitchell J, Formenti SC, DeWyngaert JK. Interfraction and intrafraction setup variability for prone breast radiation therapy. Int J Radiat Oncol Biol Phys. 2010; 76:1571–1577.

20. Kahan Z, Csenki M, Varga Z, Szil E, Cserhati A, Balogh A, et al. The risk of early and late lung sequelae after conformal radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys. 2007; 68:673–681.
21. Saliou MG, Giraud P, Simon L, Fournier-Bidoz N, Fourquet A, Dendale R, et al. Radiotherapy for breast cancer: respiratory and set-up uncertainties. Cancer Radiother. 2005; 9:414–421.
22. Smith RP, Bloch P, Harris EE, McDonough J, Sarkar A, Kassaee A, et al. Analysis of interfraction and intrafraction variation during tangential breast irradiation with an electronic portal imaging device. Int J Radiat Oncol Biol Phys. 2005; 62:373–378.

23. Baroni G, Ferrigno G, Orecchia R, Pedotti A. Real-time opto-electronic verification of patient position in breast cancer radiotherapy. Comput Aided Surg. 2000; 5:296–306.

24. Richter A, Sweeney R, Baier K, Flentje M, Guckenberger M. Effect of breathing motion in radiotherapy of breast cancer: 4D dose calculation and motion tracking via EPID. Strahlenther Onkol. 2009; 185:425–430.
25. Berbeco RI, Pope CJ, Jiang SB. Measurement of the interplay effect in lung IMRT treatment using EDR2 films. J Appl Clin Med Phys. 2006; 7:33–42.

26. Court LE, Seco J, Lu XQ, Ebe K, Mayo C, Ionascu D, et al. Use of a realistic breathing lung phantom to evaluate dose delivery errors. Med Phys. 2010; 37:5850–5857.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download