INTRODUCTION
Pancreaticopleural fistula (PPF) is a fistulous connection between the pancreas and pleural space due to prolonged chronic pancreatitis (CP). PPF is a very rare complication which presents in 0.4% of CP cases. PPF is known to occur mostly (~95%) among adults between 20 and 60 years of age and is rarely seen in pediatric pancreatitis, possibly due to the fact that CP is rare among pediatric pancreatitis [
12]. PPF in adolescence has previously been reported, but most reports were adult cases [
34]. Reports of PPF cases in children, especially before adolescence, are extremely rare.
Initial presentation of PPF is usually nonspecific chest symptoms, making it difficult to diagnose PPF and the underlying pancreatitis. While elevated serum amylase and lipase are important for diagnosis of pancreatitis, pleural fluid amylase level is important for PPF diagnosis. Differential diagnosis of elevated pleural fluid amylase includes esophageal perforation and neoplasm, but can usually be distinguished from clinical settings and imaging studies [
5].
PPF can be treated with medical, endoscopic, and surgical management. Treatment of childhood PPF with surgical intervention has already been reported [
3]. We report the first case of PPF, which was successfully managed with endoscopic stent placement, in a child with hereditary pancreatitis (HP) in Korea.
CASE REPORT
A 38-month-old boy was brought to the Seoul National University Children's Hospital due to a sudden onset of respiratory difficulty. The patient had no perinatal problems but began to develop intermittent alcoholic stool at 1 year of age. Beginning at 2 years of age, the patient suffered from frequent abdominal cramps. The patient presented with dyspnea and tachypnea but did not show signs of fever, cough, cyanosis, chest pain, vomiting, or abdominal pain.
The patient's height and weight were 95.6 cm (25–50th percentile) and 13.3 kg (10–25th percentile), respectively. His vital signs were as follows: blood pressure, 123/72 mmHg; pulse rate, 152 beats/min; respiratory rate, 36 breaths/min; and body temperature, 36.9°C. The chest wall expanded symmetrically with chest retraction. Decreased breathing sound was heard in the left lung field.
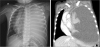
Chest radiography and computed tomography (CT) showed massive left pleural effusion (
Fig. 1). Percutaneous catheter drainage (PCD) was conducted and pleural effusion was drained. Pleural fluid revealed elevated amylase levels of 25,460 U/L and lipase levels of >6,000 U/L. Serum results also revealed elevated amylase levels of 888 U/L and lipase levels of 1,067 U/L, which was suggestive of acute pancreatitis.
Fig. 1
Chest radiography (left) and computed tomography (right) images showing massive left-sided pleural effusion.
Abdomen CT showed retroperitoneal fluid collection, diffuse pancreatic ductal dilatation, and atrophy in the pancreatic body and tail parenchyma, which was suggestive of CP. Although the abdomen CT did not reveal a definite PPF tract, the elevated amylase and lipase levels from the pleural fluid were suggestive of a fistula tract between the pancreas and pleura. Magnetic resonance cholangiopancreatography (MRCP) revealed suspicious luminal narrowing of the pancreatic duct, with probable pancreatic duct stone and sludge. A tract near the left diaphragm is thought to be the fistula that connects to the pleural cavity (
Fig. 2).
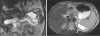
Fig. 2
Magnetic resonance cholangiopancreatography image showing suspicious narrowing of the proximal main pancreatic duct, probably due to a stone or sludge. A tract near the left diaphragm is thought to be the fistula that connects to the pleural cavity (arrow).
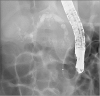
The patient was kept under fasting conditions with massive intravenous hydration, and octreotide continuous infusion and total parenteral nutrition were initiated. Endoscopic retrograde cholangiopancreatography (ERCP) was performed to further clarify the possible duct anomaly and subsequent treatment. The pancreatic duct was tortuous and dilated, and there was a filling defect due to the pancreaticolith (
Fig. 3). A single pigtail plastic stent was inserted at the pancreatic duct and some white stones and protein plugs were removed. After symptoms improved, the PCD was removed and octreotide was tapered.
Fig. 3
Endoscopic retrograde cholangiopancreatography image showing a tortured pancreatic duct stricture due to chronic pancreatitis. A single pigtail plastic stent (5 Fr, 5 cm) was inserted through the minor papilla.
The pancreatic stent was removed spontaneously via defecation 8 days after stent insertion. After 3 days, a fever of up to 39.4°C developed and left pleural effusion increased. Serum amylase and lipase levels were elevated to 285 U/L and 527 U/L, respectively. Medical treatment, including fasting and octreotide continuous infusion, was restarted, and PCD insertion was conducted again. A second ERCP was done, and the stent was placed at the minor papilla through the major papilla. The pleural drain was removed after a week of post-stent insertion with no recurrence or effusion. The patient was discharged at hospital day 32. The stent was removed after 3 months. The patient is being followed-up regularly for exocrine dysfunction and recurrence of pancreatitis by monitoring fecal elastase, and serum amylase and lipase levels, respectively.
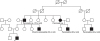
This patient had a family history of pancreatic diseases (
Fig. 4). The patient's uncle underwent 80% distal pancreatectomy due to CP with multiple stones, and the grandfather had pancreatic cancer. The great-aunt and first cousin also had pancreatic diseases. The patient and his family underwent genetic tests, which revealed that the patient, mother, uncle, and grandfather were R122H heterozygotes for
PRSS1 gene which encodes cationic trypsinogen. His father and brother had negative results.
Fig. 4
Family pedigree of the patient. The patient's uncle underwent 80% distal pancreatectomy due to chronic pancreatitis with multiple stones, and the grandfather had pancreatic cancer. The great-aunt and first cousin also had pancreatic diseases.
DISCUSSION
CP can be diagnosed when there is irreversible change to the structure of the pancreas such as sclerosis, destruction, pancreatic duct obstruction with elevated serum lipase or amylase levels, and exocrine/endocrine pancreatic insufficiency. The incidence of CP was 9.62 cases per 100,000 person-years and mortality from CP was 0.09 per 100,000 person-years [
6]. There is no research on the epidemiology of pediatric-specific CP. In studies comparing incidence rates by age from 0–34 years and <20 years, it was reported as low as 0.5 per 100,000 people [
7]. The incidence rate is proportional to age and the rate in adults is 4–9 times higher than in pediatrics [
8].
In contrast with adult CP, in which smoking and alcohol are important risk factors, genetic predisposition is a major cause of CP in children [
9]. If pediatric pancreatitis recurs or appears to be chronic, a thorough family history should be conducted and the possibility of HP should be considered [
2]. If there are three or more patients with pancreatitis in the second generation of the same household, it is necessary to suspect HP and perform genetic screening. Mutations such as the
PRSS1 gene, CF transmembrane regulator (
CFTR) gene, serine peptidase inhibitor Kazal type 1 (
SPINK1) gene, and chymotrypsin C (
CTRC) gene are well-known to cause HP [
10]. The most representative of these are mutations of R122H and N291 in exon 3 and exon 2, respectively, of the
PRSS1 gene. Shin et al. [
11] reported N291 mutations in HP for the first time in Korea. This case also had a family history of pancreatic disease, and an R122H mutation of the
PRSS1 gene was revealed. Although the patient's mother also had a PRSS1 gene mutation, she did not show any symptoms because the
PRSS1 penetrance is about 78% [
12]. Regular outpatient clinic follow-up is recommended as HP patients have higher incidences of exocrine and endocrine failure, diabetes mellitus, and pancreatic cancer [
13].
PPF is developed through the leakage of pancreatic fluid as a result of pancreatic duct disruption [
1]. When a pancreatic duct or pseudocyst is connected to the pleural cavity, PPF forms [
14]. This could result in massive pleural effusion, which may initially present as respiratory symptoms such as dyspnea.
Diagnosis of PPF is established by thoracocentesis and ascertaining the elevation of amylase in the pleural fluid. However, pleural effusion due to PPF is rare. The most common cause of amylase-elevated pleural effusion is malignancy, such as lung cancer, leukemia, and metastatic tumors. In addition, liver cirrhosis, tuberculosis, and hydronephrosis also need to be distinguished [
4]. Imaging techniques such as CT, MRCP, and ERCP can be used to confirm the fistulous tract between the pancreas and pleural space [
15]. Since MRCP is more sensitive than CT and less invasive than ERCP, it is used as the imaging technique of choice [
16]. MRCP also found the tract estimated to be the fistula in our case.
PPF treatment can be divided into medical, endoscopic, and surgical methods. Due to the scarcity of PPF, there is no randomized study on treatment comparison. Therefore, there is not a consensus about which treatment is superior. If there is no anomaly of the pancreatic duct or the anomaly is mild, medical treatment is recommended initially. The medical approach consists of fasting and total parenteral nutrition for 2–3 weeks, and octreotide administration to suppress pancreas exocrine function. These allow the pancreas to rest and close the fistulous tract [
5]. If there is a large amount of pleural effusion and symptoms, drainage via the chest tube is helpful. In 30–60% of cases, medical treatment is successful, but endoscopic and surgical treatment can also be attempted. In the presence of ductal stenosis, an endoscopic approach can be used initially by placing a stent or balloon dilatation, due to its minimal morbidity and mortality. Stent insertion reduces intraductal pressure and leakage. Endoscopic intervention is generally known to close the fistula within 2–3 weeks in 48% of patients, and the stent usually lasts from 4 weeks to 6 months [
14]. Although the outcomes of the endoscopic approach are not often reported, one study reported that initial resolution of the duct disruption was achieved in 88% of patients and that fluid collection was reduced by 85% [
17]. If stent placement is not possible due to severe duct obstruction or a distally located fistula, or if there is no response to medical management for 3–4 weeks, surgery such as distal pancreatectomy or pancreaticojejunostomy can be considered [
918]. Operative mortality is less than 5%, and recurrence is about 11%. Long-term prognosis of PPF is known to be good in 80–95% of cases. The total mortality of PPF is about 5% [
19].
We described the case of a 3-year-old child who presented with massive pleural effusion due to PPF, which was successfully managed with endoscopic and conservative management for the first time in Korea. Although CP is scarce in children, the patient had a genetic predisposition towards CP, ultimately leading to PPF. Obtaining a detailed history, including family history and accompanying symptoms, in patients with pleural effusion is important in reducing misdiagnosis. In addition, if a child with CP has a family history of pancreatitis, genetic screening (including PRSS1) should be performed. PPF should be suspected when fluid amylase levels are high in patients with pleural effusion and can be treated successfully by conservative and endoscopic treatment.







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download