This article has been
cited by other articles in ScienceCentral.
Abstract
We report a case of a 13-year-old girl who presented with a 2-month history of intermittent abdominal pain. Laboratory examination showed hepatitis and pancreatitis. Because of persistent vomiting, computed tomography (CT) was performed, which revealed a circumferential soft tissue density in the duodenal wall, causing partial obstruction. Supportive therapy failed. Repeat CT showed no significant change from the initial study. The patient underwent upper endoscopy, which revealed a mass in the second portion of the duodenum, which occluded most parts of the lumen. The histopathological finding was consistent with an anaplastic large cell lymphoma, a rare form of small bowel neoplasm. After the third course of chemotherapy, complete resolution of the mass was noted, and her symptoms were relieved.
Keywords: Hepatitis, Pancreatitis, Vomiting, Duodenal mass
INTRODUCTION
Gastrointestinal (GI) tract neoplasms constitute 2–6% of all neoplasms in adults. Small bowel neoplasms are rare and more so in children [
1]. Patients may present with nonspecific GI symptoms, including abdominal pain (67%), intestinal obstruction (30%), GI bleeding (24%), and bowel perforation (12%) [
2]. Differentiating between neoplastic and non-neoplastic conditions from computed tomography (CT) findings can be challenging in clinical practice. We report a case of a patient who presented with nonspecific abdominal symptoms that initially had a presumed diagnosis of a non-neoplastic condition from the initial CT result but eventually diagnosed as a proximal small bowel neoplasm from more advanced investigations. After obtaining an informed consent, we received an approval from the institutional research committee (ID 06-61-166) to report this case.
CASE REPORT
A previously healthy 13-year-old girl presented to our hospital with a 2-month history of fatigue and intermittent abdominal pain. She had intentionally attempted to lose 5 kg of weight but denied history of fever, vomiting, change in bowel habits, or trauma. Physical examination did not reveal abdominal tenderness, mass, or organomegaly. Initial liver function tests showed alkaline phosphatase level of 352 U/L, aspartate transaminase level of 828 U/L, alanine transaminase level of 1,062 U/L, gamma-glutamyl transferase level of 321 U/L, total bilirubin level of 0.3 mg/dL, direct bilirubin level of 0.2 mg/dL, and serum albumin level of 32 g/dL. Complete blood count, viral hepatitis panel, and ceruloplasmin and autoimmune hepatitis markers were unremarkable. Two days after admission, the patient developed severe abdominal pain with elevated serum amylase and lipase levels. Upper abdominal ultrasonography revealed mild parenchymatous liver disease with prominent size of the visualized portions of the pancreas and dilated common bile duct without peripancreatic fluid collection, abscess, stone, or mass. After 48 hours of supportive management, abdominal pain and non-bilious vomiting persisted. CT of the upper abdomen did not reveal evidence of complications of acute pancreatitis but demonstrated a circumferential soft tissue density along the wall of the second and third portions of the duodenum with proximal small bowel dilatation (
Fig. 1A). The initial impression was of an intramural duodenal hematoma (IDH). CT also showed upstream dilatation of both the common bile and pancreatic ducts compatible with distal obstruction and probably causing the pancreatitis. Enteral feeding was gradually advanced successfully.
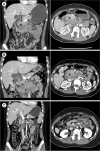 | Fig. 1 (A) Computed tomography revealed circumferential soft tissue density (*) along the wall of the second and third parts of the duodenum with proximal bowel dilatation and upstream dilatation of both common bile (ⱡ) and pancreatic ducts. (B) The follow-up scan showed no significant change in circumferential soft tissue density (*) after 17 days. (C) Complete resolution of the mass within 4 months after the third course of chemotherapy.
|
Five days after hospital discharge, the patient had recurrent postprandial vomiting. Follow-up abdominal CT, 17 days after the initial one, showed no significant changes in the circumferential soft tissue density; therefore, a neoplasm rather than IDH was suspected (
Fig. 1B). Magnetic resonance cholangiopancreatography revealed a soft tissue mass along the duodenal wall with multiple lymph nodes in the mesenteric regions <1 cm in size, indicating a possible neoplasm or inflammatory pseudotumor. Esophagogastroduodenoscopy revealed an intraluminal mass in the second portion of the duodenum, which occluded 80% of the lumen, without ulceration or exudates (
Fig. 2A, B). The histopathological finding of the mass was consistent with anaplastic large cell lymphoma. Bone marrow aspiration, CT scan of the chest showed no evidence of metastasis. In the following four months, she received three courses of chemotherapy. Subsequently, CT revealed complete resolution of the duodenal mass (
Fig. 1C) with complete luminal patency of the duodenum and macroscopically normal mucosa (
Fig. 2C, D). The patient recovered well without recurrence at 12 months of follow-up.
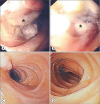 | Fig. 2 (A, B) Endoscopic examination revealed that mass (*) at the second part of the duodenum occluded approximately 80% of the lumen without ulceration or exudates. (C, D) Complete patency of the similar area of the duodenum 4 weeks after the completion of chemotherapy.
|
DISCUSSION
Our patient had an initial CT result suggestive of IDH that was believed to follow a recent episode of acute pancreatitis, which subsequently caused significant obstruction of the pancreatobiliary system, a condition that has been reported in a previous adult study [
3]. However, in the absence of history of abdominal trauma, IDH is extremely rare. With regard to the small bowel wall thickening, short length, asymmetrical irregular wall thickness, and solid homogeneous or heterogeneous contrast-enhancing pattern with peri-enteric fat stranding may be observed in small bowel neoplasm, similar to those in the abovementioned patient. However, magnetic resonance imaging, not CT, is the recommended diagnostic modality to evaluate soft tissue in the GI tract [
4].
For small bowel neoplasms, the typical appearance of GI lymphoma shows coarse segmental wall thickening with ulceration or necrosis and usually demonstrates bulky lymphadenopathy [
4]. The most common GI segments involved in lymphoma are the stomach and small bowel (with the ileum being the most common site [65%], followed by the jejunum [25%] and duodenum [10%]). The diagnostic criteria in GI lymphoma include the following: 1) absence of palpable superficial lymphadenopathy; 2) no evidence of enlarged mediastinal lymph nodes; 3) normal total and differential white blood cell count; 4) predominance of bowel lesions at laparotomy with only lymph nodes obviously affected in the immediate vicinity; and 5) absence of tumor involvement in the liver and spleen [
5]. According to the World Health Organization classification, anaplastic large cell lymphoma classified as peripheral T-cell lymphoma is divided into anaplastic lymphoma kinase (ALK) -positive and ALK-negative. ALK-positive subtype occurs more frequently in younger patients with a favorable outcome responsive to treatment [
6].
Our case highlights the fact that distinguishing between neoplastic and non-neoplastic conditions from CT findings in the context of GI obstruction can be difficult. A more advanced imaging study, such as magnetic resonance imaging and endoscopy, may be helpful in reaching a diagnosis.
ACKNOWLEDGEMENTS
Pornthep Tanpowpong is the recipient of the Research Career Development Award from the Faculty of Medicine, Ramathibodi Hospital, Mahidol University.
References
1. North JH, Pack MS. Malignant tumors of the small intestine: a review of 144 cases. Am Surg. 2000; 66:46–51.
2. Farhat MH, Shamseddine AI, Barada KA. Small bowel tumors: clinical presentation, prognosis, and outcome in 33 patients in a tertiary care center. J Oncol. 2008; 2008:212067.

3. Ma JK, Ng KK, Poon RT, Fan ST. Pancreatic-induced intramural duodenal haematoma. Asian J Surg. 2008; 31:83–86.

4. Fernandes T, Oliveira MI, Castro R, Araújo B, Viamonte B, Cunha R. Bowel wall thickening at CT: simplifying the diagnosis. Insights Imaging. 2014; 5:195–208.

5. Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011; 17:697–707.

6. Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. International Peripheral T-Cell Lymphoma Project. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008; 111:5496–5504.






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download