II. Materials and Methods
The study was carried at Department of Oral and Maxillofacial Surgery, Faculty of Dental Sciences, SGT University (Gurgaon, India) from August 2010 to December 2011. The study was approved by institutional review board of SGT Dental College (SGTDC/PPL/Com/EC/09/2010). Written informed consent was taken from all the patients.
This study was conducted on 20 patients aged 15 to 30 years who needed similar bilateral extractions in the mandible. Patient selection was done based on inclusion criteria such as adequate patient availability, compliance with instructions and follow up, proper oral hygiene maintenance, and medical fitness to undergo a minor surgical procedure.
All the extractions were done atraumatically by the same operator under local anesthesia (lignocaine 2% with adrenaline 1:200,000). One extraction socket (right or left) in each patient was selected randomly for treatment with PRGF (prepared according to the protocol described below) and covered with an autologous fibrin, and the other socket was left to heal normally. Bone regeneration was evaluated 13 weeks post extraction using cone-beam computed tomography (CBCT) and considering various parameters.
Method for PRGF preparation is as follows.
1) Blood (10 mL) were drawn from the patient's anticubital fossa and transferred in vaccutubes containing 3.2% sodium citrate (anticoagulant).
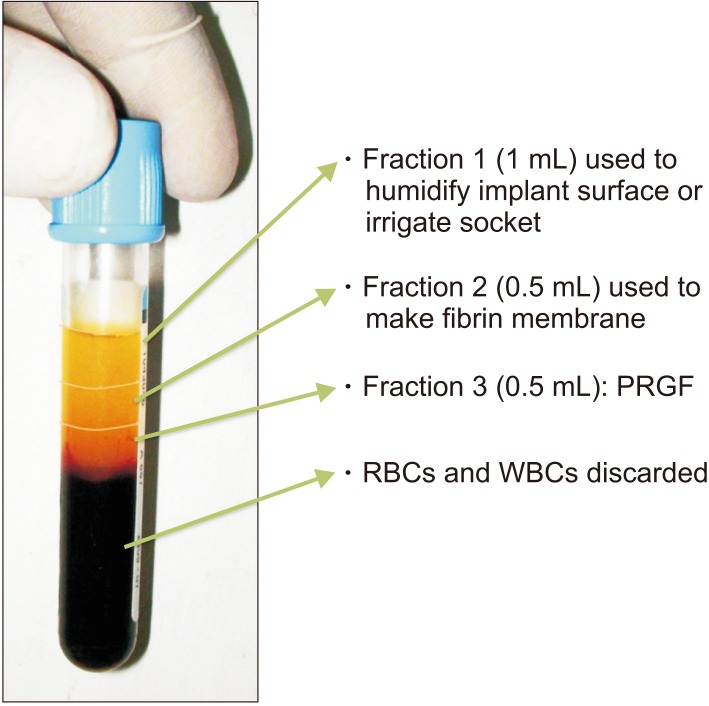
2) The vaccutubes were centrifuged for a preset cycle of 10 minutes to separate the blood components as red blood cells (RBCs; red color, bottom half), white blood cells (WBCs; a thin white colored band) and plasma (straw colored, top half). (
Fig. 1). The plasma fraction used in the growth factor (GF)-assisted regenerative technique was restricted to the 1 mL volume immediately above the RBC line of the tube.
3) This plasmatic component was divided into three plasmatic fractions arranged by molecular weight. In descending order, these fractions were in
Fig. 1.
(1) Fraction 1: Plasma poor in GFs, in the top 1 mL layer
(2) Fraction 2: Plasma with GFs, in the next 0.5 mL layer
(3) Fraction 3: Plasma rich in GFs (PRGF), in the bottom 0.5 mL layer
4) Using micropipettes, these components were separated out into glass dishes.
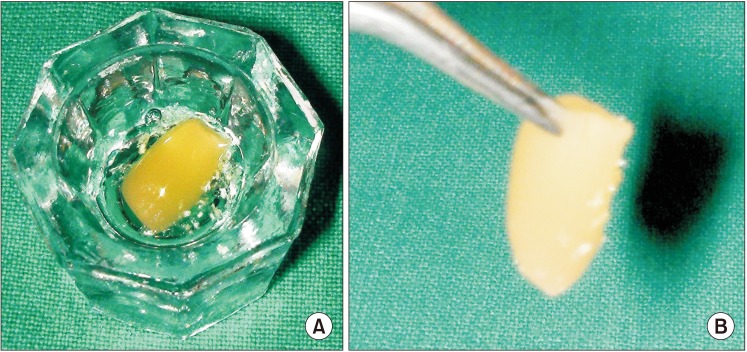
5) Fraction 3 was activated with calcium chloride (50 µL per mL) and left undisturbed for 8 to 10 minutes, during which platelet dehiscence occurred, leading to the release of the GFs. By placing the precipitate in a thermal unit, we reduced the gelation period to 3 minutes.
6) The activated PRGF gel was easily carried with forceps (
Fig. 2) to the extraction sockets.(
Fig. 3)
The data was entered in Microsoft Excel Sheet (Microsoft Excel 2010; Microsoft, Redmond, WA, USA) and analyzed using PASW Statistics 18 (IBM Corp., Armonk, NY, USA) for relevant statistical comparison.
IV. Discussion
Due to their potential therapeutic use, growth factors, proteins that have an essential role in the healing process and tissue formation, have generated considerable interest during the past few years. Their mode of action is to bind to the extracellular domain of a target receptor, thereby activating an intracellular signaling cascade
8. Growth factors trigger biological effects such as direct cell margination (chemotaxis), angiogenesis, cellular proliferation, and differentiation, all of which are key events in repair and regeneration processes. They are located in blood plasma and platelets in high concentrations
9.
Since 1990, many authors have explored specific sequestration and concentration processes of autologous platelets in platelet-rich plasma (PRP) and have studied the growth factors present within them. The literature contains reports of a variety of platelet-derived products that are supposed to enhance bone regeneration. The different terminologies and variety of preparation methods can confuse beginners, so it is important to understand the basic differences among the most commonly used platelet derivatives.
PRP was first described by Marx and colleagues
1011. It is a suspension of platelets in plasma that has a higher platelet concentration than the original blood from which it is prepared. Marx and colleagues
1011 first documented the use of PRP to increase the concentration of GFs and the potential of PRP to increase the rate of bone formation. In his study, he concluded that PRP contains a high concentration of growth factors and quantifiably enhances bone regeneration
10. Marx et al.
11 thus deemed PRP to be a practical tool for enhancing the rate of bone formation and the final quantity of bone formed because of its concentration of platelets and GFs.
To prepare PRP using the Marx protocol, autogenous whole venous blood is withdrawn. To achieve anticoagulation, 1 mL of citrate phosphate dextrose is added to 5 mL of blood. The blood is then centrifuged at 5,600 rpm in an Electro Medics 500 gradient density cell separator (Medtronic, Minneapolis, MN, USA), which separates the blood into three components—RBCs, PRP (also called the buffy coat), and PPP (platelet poor plasma). Because these components have different densities, the PPP layer forms at the top, the PRP layer forms in the middle, and the RBC layer is at the bottom. The PPP layer at the top is separated out, and the rest of the contents are centrifuged again at 2,400 rpm. This fully separates the PRP from the RBCs. Before use, the PRP is activated by initiating the coagulation process, which converts it into a gel form that can be handled easily. For this, a mixture of 10 mL of 10% calcium chloride is mixed with 10,000 units of topical bovine thrombin. Then, 1 mL of that mixture, 6 mL of PRP, and 1 mL of air are agitated together in a 10 mL syringe for 6 to 10 seconds. The gelled PRP is now ready to be used
11.
Studies by Sammartino et al.
12 and Célio-Mariano et al.
13 support the use of PRP in third molar surgery. Randomized clinical trials conducted by Célio-Mariano et al.
13, Alissa et al.
14, and Antonello et al.
15 show that bone regeneration in extraction sockets saw statistically significant enhancement when PRP was used. PRP augments physiologic healing and regenerative processes without interfering with the hemostatic balance, unlike existing synthetic biomaterials. It is cost effective, can be prepared on-site, and has an extremely low risk of infection transmission because it is autologous
16.
In 1994, Muntean et al.
17 reported a clinical concern about the use of PRP: some orthopedic, neurosurgical, and cardiovascular surgical patients in whom bovine thrombin was used as a hemostatic agent developed bleeding episodes later. It has also been suggested that PRP could promote infections, with an incidence of 2.0% to 3.5%. Another drawback of PRP is that its preparation requires two centrifugation procedures, which means the processing time is relatively long. Marx
10 suggested that autologous growth factors be extracted from PRP using two different devices and two centrifugation cycles. Studies by Weibrich et al.
18 documented the inadequacy of those devices. Many studies have questioned the actual effectiveness of PRP because the rapid release of growth factors produces immediate but transitory effects that might prove insignificant for tissue repair over time. According to Gürbüzer et al.
19, PRP might not lead to increased bone healing after impacted third-molar surgery.
The disadvantages of PRP are that the preparation method is technique sensitive and time consuming (usually at least 30 minutes) and uses bovine thrombin for activation, which can lead to the transmission of unknown infections from animalderived biologicals
16.
To overcome those disadvantages, Anitua et al.
48 developed PRGF, which is described in the Materials and Methods section. It is purely autologous because calcium chloride is used for activation instead of bovine thrombin, and it requires only one cycle of centrifugation.
Nishiyama et al.
20 found that PRGF preparation almost completely eliminates both RBCs and WBCs and concentrates the platelets 2.84 fold, whereas PRP preparation concentrates platelets and WBCs by 8.79 and 5.51 fold, respectively. They explained that PRGF is characterized by a moderate concentration of platelets and the elimination of leucocytes. PRGF thus avoids the potential pro-inflammatory effects of proteases and acid hydrolases, both components of WBCs. They found that PRP preparations significantly suppressed cell growth at high doses
in vitro , making PRGF more appropriate for use in tissue regeneration.
Platelet rich fibrin (PRF) is a fibrin network that contains platelets, WBCs, serum, and concentrated growth factors. PRF was developed by Choukroun et al.
21 to eliminate xeno factors, i.e., the bovine thrombin used in PRP activation. They achieved clotting by stimulating only an endogenous coagulation pathway, making PRF a self-clotted preparation of a PRP derivative. Because of the simplicity of its preparation protocol, PRF is considered to be a PRP substitute in regenerative medicine. Its preparation requires neither an anticoagulant nor a coagulant, and the reduced centrifugation and eliminated fractionation reduce the time required for its preparation compared with PRP. To prepare PRF, blood samples are collected without anticoagulants and centrifuged to form a fibrin clot that is a reservoir of platelets, leucocytes, cytokines, and immune cells. It allows slow release of cytokine-transforming growth factor, platelet-derived growth factor, vascular endothelial growth factor, and epidermal growth factor, all of which play important roles in angiogenesis, tissue healing, and cicatrization. However, a recent systematic review and meta-analysis found that PRF has no positive effect on bone healing after the extraction of impacted mandibular third molars
22.
Advanced-platelet rich fibrin (A-PRF) is a modification of PRF developed by Choukroun
23. The use of low-speed centrifugation forms a fibrin clot that is softer and contains more WBCs than previous methods.
Concentrated growth factor (CGF) is another modified form of PRF that is prepared by repeatedly switching the centrifugation speed which produces a fibrin clot that is relatively stiffer than that of A-PRF
24. According to Masuki et al.
24, both A-PRF and CGF are more potent than PRP.
Kawase
16 explained the differences among various platelet derivatives, stressing the importance of standardizing the preparation protocols for PRP and PRGF to enable appropriate comparisons of clinical data among international laboratories and investigators.
Researchers have tried to classify platelet products. Dohan Ehrenfest et al.
25 in 2009 classified these products into four groups by their leucocyte and fibrin content: pure platelet rich plasma (P-PRP), Vivostat PRF or Anitua's PRGF leucocytes and PRP (L-PRP), pure platelet rich fibrin (P-PRF), and leucocyte and platelet rich fibrin (L-PRF) also called Choukroun's PRF
25. Recently, Magalon et al.
26 published the DEPA classification for PRP—dose of injected platelets, efficacy of production, purity of the PRP, and activation of the PRP.
PRGF obtains growth factors and plasma proteins from a patient's own blood shortly before its therapeutic use to accelerate the repair and regeneration mechanisms of various tissues
7. Because its mechanisms of action affect basic cellular and molecular processes common to all tissues, PRGF has a wide range of applications
27. It releases growth factors that promote healing, reduce the inflammatory response, and accelerate osseous regeneration
28. Also, the plasma fraction with the smallest platelet content can be used to obtain an autologous fibrin plug that serves as a biomaterial barrier to seal post-extraction sites
4.
PRGF can be obtained from small volumes of extracted blood, as little as 5 mL. It can be easily and reproducibly prepared in an office environment in 15 to 20 minutes. It has no antigenic effects. It is the first and only described technique that produces only platelets, excluding leukocytes and eliminating inflammatory interleukins of leukocytal origin. The system and protocol are simple, user-friendly, and affordable.
We obtained approximately 1 mL of PRGF from 8 mL of each patient's blood in our office. With a 10-minute single cycle of centrifuge (
Fig. 6), preparation was quick and easy. This PRGF gel (which is a coagulated mass) is easy to manipulate, but it must be applied without delay to preserve the growth factor activity. We observed that both the control and PRGF sites showed good bone quality, but the PRGF sites showed better bone quality and quantity than the control sites. In the randomized controlled trial conducted by Anitua et al.
29, the healing of extraction sockets in the treatment group was better than in the control group clinically, radiographically, and histopathologically. Those results confirmed his 1999 histopathological study
27 of bone growth with PRGF. He found 100% epithelization in all cases and significantly better osseous regeneration (in terms of both quantity and quality) with PRGF than without it
27.
Anitua et al.
29 reported that the mean bone densities of the PRGF and control groups were 450 and 318 HU, respectively, after 10 to 12 weeks
29. In another study, Anitua et al.
4 found that 11 post-extraction sites filled with PRGF showed a sufficient quantity (more than 500 HU) and quality of bone (type II) in 8 to 12 weeks. In our study, the mean bone density in the PRGF group after 13 weeks was 647.95 HU, which was appreciably higher than in the non-PRGF group (500.05 HU).
These results suggest that reinforcing the growth factor concentration by applying PRGF to the wound improves bone regeneration, which is in agreement with several preclinical animal studies that found the bone regeneration effect of PRGF to be highly consistent
1730. This effect might be explained in part by the mitogenic and proliferative effects that some of the growth factors released by PRGF have on osteoprogenitor cells. It has been fully demonstrated that growth factors derived from platelets can stimulate the proliferation of different cells, including human trabecular bone cells, human osteoblast-like cells, human stromal stem cells, and human mesenchymal stem cells
3132.
In 1985, Lekholm and Zarb
3 proposed a classification of bone quality in terms of density. Based on its radiographic appearance and resistance at drilling, they classified bone quality using four categories: Type 1, Bone composed almost entirely of homogenous compact bone; Type 2, Bone composed of a thick layer of compact bone surrounding a core of dense trabecular bone; Type 3, Bone composed of a thin layer of cortical bone surrounding a core of dense trabecular bone; and Type 4, Bone composed of a thin layer of cortical bone surrounding a core of low-density trabecular bone of poor strength.
Anitua et al.
4 proposed a new classification using average bone density measured in Hounsfield units: Bone type I, 1,000-1,600 HU; Bone type II, 600-1,000 HU; Bone type III, 300-600 HU; Bone type IV, 100-300 HU; and Bone type V, <0-100 HU.
In our study, the PRGF sites were characterized by type II bone (mean, 647.95 HU), whereas the control sites were characterized by type III bone (mean, 500.05 HU). Our results thus confirm that PRGF induces excellent regenerative activity in the extraction socket, which attests to its therapeutic potential.
Our findings raise a question about follow-up time. Anitua et al.
4 conducted bone density scans 10 weeks and 16 weeks post-extraction and found similar bone density values inside the alveolus at both times. Therefore, we should not expect improvements after longer periods of time. However, the effect of PRGF in a short time is one of its major advantages for patients. Because Anitua et al.
4 reported no significant difference between 10 and 16 weeks, we conducted CBCT only once at 13 weeks because we did not want to expose our patients to radiation twice.
The data presented in this study confirm that the PRGF technique represents a great advance for both immediate and delayed short- and midterm procedures by allowing a drastic reduction in waiting time between surgeries. Without PRGF, post-extraction sites usually require 12 months to heal completely. Therefore, PRGF not only shortens the time between surgeries, it improves patient function and aesthetics and the predictability of future treatments.
In this study, none of the patients reported pain, inflammation, or infection in the extraction sockets filled with PRGF throughout the follow up period. The use of autologous PRGF does not cause any side effects; it's a safe and simple procedure to follow and is inexpensive and efficacious for patients.
Our 20 patients had bone density of more than 500 HU in most of the extraction sockets receiving PRGF (type II and type III bone, as evaluated using CBCT scans) because of the influence of the growth factors in the PRGF. The technique for processing PRGF was easier and simpler than the techniques previously used for PRP preparation. The disadvantages of PRGF were its placement time (it should be placed within 8 hours of activation) and the limitation that 1 mL of PRGF contain no more than 50 µL of calcium chloride to prevent damaging the growth factors. In a recent article, Anitua et al.
33 suggested a further reduction in the amount of anticoagulant and activator used in PRGF preparation.
We have shown that PRGF can promote the growth of Type II bone, which is considered ideal for implant placement, in a shorter period than seen with ordinary healing, which can shorten the wait times for patients who require post-extraction implant placement. PRGF can also be used to speed the healing of bone defects such as cystic cavities, for which primary closure is done after removing the cystic lining. When used in the sockets of orthodontic extractions, PRGF could allow the orthodontist to start moving the adjacent teeth sooner than would otherwise be advisable.
Mozzati et al.
34 evaluated the efficacy of PRGF in healing post-extraction sockets in patients affected by insulin-dependent diabetes mellitus, and they concluded that it improved and accelerated the healing process. Haraji et al.
35 concluded that PRGF significantly reduced the incidence of alveolar osteitis (dry socket) after surgical removal of the third molars. PRGF has also been used in sinus lift procedures
36. Clearly, the future appears promising for PRGF.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download