II. Materials and Methods
The present retrospective study includes 50 cases of formalin fixed and paraffin embedded tissues of OSCC and corresponding LNs from neck dissections. Tissue blocks were retrieved from archives of the Department of Oral Pathology and Microbiology, KLE VK Institute of Dental Sciences (Belagavi, India) from October 2015 to September 2017. Tissue sections of 4 µm thickness of all levels of LNs were obtained for a total of 1,078 LNs. Tissue sections were stained with H&E and evaluated histopathologically.
Parameters and classification systems follow:
1) Clinical parameters: Demographic data for age and sex as obtained from departmental case records were tabulated.
-
2) OSCC:
(1) The grades of primary tumors were classified according to WHO Criteria into well differentiated (WD), moderately differentiated (MD), and poorly differentiated squamous cell carcinoma (PDSCC).
(2) Histopathologic features of tumor were included as follows, per Broder's and Byrne's classification: tumor grade, invasive front, neural invasion, and vascular invasion.
3) LNs: Evaluation of LNs and comparison with clinical and histopathological parameters in neck dissection cases of OSCC was obtained by consensus of three trained observers with similar experience in histopathology.
LN reactivity patterns were assessed according to Tsakraklides rule
5 into lymphocyte predominance, lymphocyte depletion, germinal center predominance, unstimulated node pattern, sinus histiocytosis, cortical hyperplasia, paracortical hyperplasia, increased vascularity, and nodal status of LN.
Clinical parameters in OSCC cases were assessed and compared with histologic parameters and LN reactivity patterns using chi-square analysis as the test of significance.
Go to :

III. Results
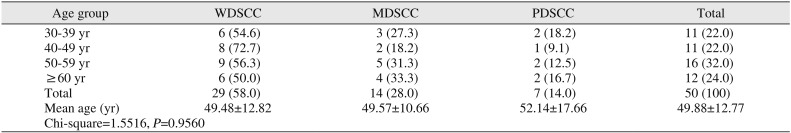
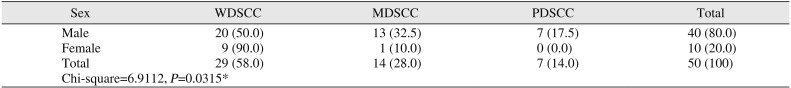
Among studied cases, the age range was 30 to 76 years. Within these age groups, 58.0% of OSCC cases were WDSCC. The male to female ratio was 4:1 in our study.(
Tables 1,
2)
Table 1
Comparison of tumor grades by age groups
|
Age group |
WDSCC |
MDSCC |
PDSCC |
Total |
|
30-39 yr |
6 (54.6) |
3 (27.3) |
2 (18.2) |
11 (22.0) |
|
40-49 yr |
8 (72.7) |
2 (18.2) |
1 (9.1) |
11 (22.0) |
|
50-59 yr |
9 (56.3) |
5 (31.3) |
2 (12.5) |
16 (32.0) |
|
≥60 yr |
6 (50.0) |
4 (33.3) |
2 (16.7) |
12 (24.0) |
|
Total |
29 (58.0) |
14 (28.0) |
7 (14.0) |
50 (100) |
|
Mean age (yr) |
49.48±12.82 |
49.57±10.66 |
52.14±17.66 |
49.88±12.77 |
|
Chi-square=1.5516, P=0.9560 |
|
|
|
|

Table 2
Comparison of tumor grades by sex
|
Sex |
WDSCC |
MDSCC |
PDSCC |
Total |
|
Male |
20 (50.0) |
13 (32.5) |
7 (17.5) |
40 (80.0) |
|
Female |
9 (90.0) |
1 (10.0) |
0 (0.0) |
10 (20.0) |
|
Total |
29 (58.0) |
14 (28.0) |
7 (14.0) |
50 (100) |
|
Chi-square=6.9112, P=0.0315*
|
|
|
|
|

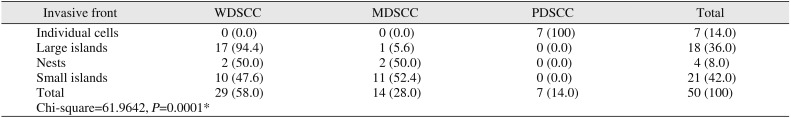
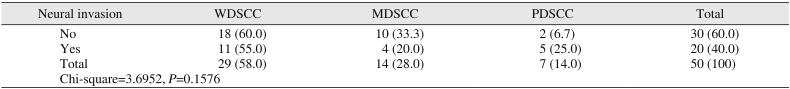
There was statistical significance observed between tumor grade and pattern of invasive front (
P=0.0001).(
Table 3) Of 50 cases, 20 cases showed neural invasion of tumor cells and 10 cases showed vascular invasion of tumor cells. However, there was no statistical significance observed between tumor grade and neural invasion. Among 10 cases that showed vascular invasion, 40% were WDSCC and PDSCC, while only 20% were MDSCC (
P<0.05).(
Tables 4,
5)
Table 3
Comparison of tumor grades to invasive front
|
Invasive front |
WDSCC |
MDSCC |
PDSCC |
Total |
|
Individual cells |
0 (0.0) |
0 (0.0) |
7 (100) |
7 (14.0) |
|
Large islands |
17 (94.4) |
1 (5.6) |
0 (0.0) |
18 (36.0) |
|
Nests |
2 (50.0) |
2 (50.0) |
0 (0.0) |
4 (8.0) |
|
Small islands |
10 (47.6) |
11 (52.4) |
0 (0.0) |
21 (42.0) |
|
Total |
29 (58.0) |
14 (28.0) |
7 (14.0) |
50 (100) |
|
Chi-square=61.9642, P=0.0001*
|
|
|
|
|

Table 4
Comparison of tumor grades with neural invasion
|
Neural invasion |
WDSCC |
MDSCC |
PDSCC |
Total |
|
No |
18 (60.0) |
10 (33.3) |
2 (6.7) |
30 (60.0) |
|
Yes |
11 (55.0) |
4 (20.0) |
5 (25.0) |
20 (40.0) |
|
Total |
29 (58.0) |
14 (28.0) |
7 (14.0) |
50 (100) |
|
Chi-square=3.6952, P=0.1576 |
|
|
|
|

Table 5
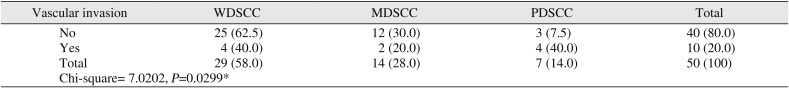
Correlation of tumor grades with vascular invasion
|
Vascular invasion |
WDSCC |
MDSCC |
PDSCC |
Total |
|
No |
25 (62.5) |
12 (30.0) |
3 (7.5) |
40 (80.0) |
|
Yes |
4 (40.0) |
2 (20.0) |
4 (40.0) |
10 (20.0) |
|
Total |
29 (58.0) |
14 (28.0) |
7 (14.0) |
50 (100) |
|
Chi-square= 7.0202, P=0.0299*
|
|
|
|
|

A total of 1,078 LNs were harvested and evaluated from 50 neck dissection cases of OSCC. LNs patterns were observed in the following order: increased vascularity in 472 LNs, lymphocyte predominance (LP) pattern in 396 LNs, germinal center predominance (GCP) pattern in 375 LNs, unstimulated node pattern (UNP) in 318 LNs, cortical hyperplasia (CH) in 299 LNs, paracortical hyperplasia (PCH) in 255 LNs, sinus histiocytosis (SH) pattern in 121 LNs, metastases in 56 LNs, and lymphocyte depletion (LD) pattern in 18 LNs.(
Fig. 1)
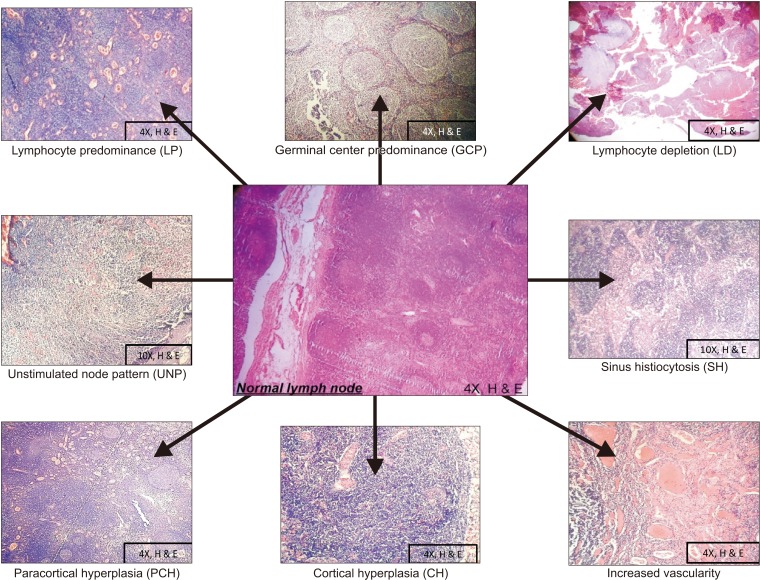 | Fig. 1Reactivity patterns of lymph node.
|
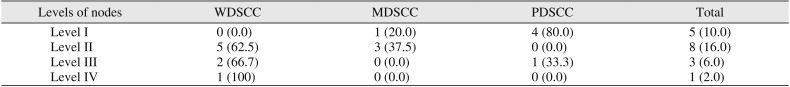
Further comparison of metastasis in each level (level I to IV) showed the most metastasis in level II LNs, which was followed by level I, level III, and level IV, in order. Interestingly, 80% of PDSCC cases with metastasis was in level 1 LNs.(
Table 6)
Table 6
Comparison of tumor grades with positive nodes by level
|
Levels of nodes |
WDSCC |
MDSCC |
PDSCC |
Total |
|
Level I |
0 (0.0) |
1 (20.0) |
4 (80.0) |
5 (10.0) |
|
Level II |
5 (62.5) |
3 (37.5) |
0 (0.0) |
8 (16.0) |
|
Level III |
2 (66.7) |
0 (0.0) |
1 (33.3) |
3 (6.0) |
|
Level IV |
1 (100) |
0 (0.0) |
0 (0.0) |
1 (2.0) |

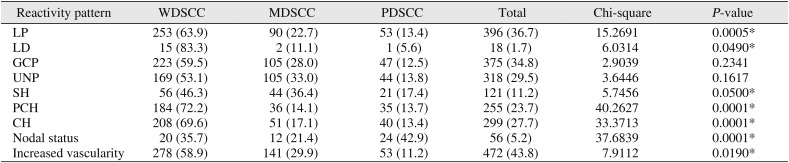
Of 396 LNs with LP pattern, 63.9% LP pattern LNs were in cases of WDSCC, 22.7% LP pattern LNs were in cases of MDSCC, and 13.4% pattern LNs were in cases of PDSCC. This difference between LN LP pattern and tumor grade was statistically significant (
P=0.0005). In investigation of LD pattern, 83.3% of LNs in WDSCC cases showed LD pattern, 11.1% of LNs in MDSCC cases showed LD pattern, and 1 LN in PDSCC showed LD pattern. This difference was statistically significant (
P=0.0490).(
Table 7)
Table 7
Comparison of tumor grades in OSCC and lymph node reactivity patterns (n=1,078)
|
Reactivity pattern |
WDSCC |
MDSCC |
PDSCC |
Total |
Chi-square |
P-value |
|
LP |
253 (63.9) |
90 (22.7) |
53 (13.4) |
396 (36.7) |
15.2691 |
0.0005*
|
|
LD |
15 (83.3) |
2 (11.1) |
1 (5.6) |
18 (1.7) |
6.0314 |
0.0490*
|
|
GCP |
223 (59.5) |
105 (28.0) |
47 (12.5) |
375 (34.8) |
2.9039 |
0.2341 |
|
UNP |
169 (53.1) |
105 (33.0) |
44 (13.8) |
318 (29.5) |
3.6446 |
0.1617 |
|
SH |
56 (46.3) |
44 (36.4) |
21 (17.4) |
121 (11.2) |
5.7456 |
0.0500*
|
|
PCH |
184 (72.2) |
36 (14.1) |
35 (13.7) |
255 (23.7) |
40.2627 |
0.0001*
|
|
CH |
208 (69.6) |
51 (17.1) |
40 (13.4) |
299 (27.7) |
33.3713 |
0.0001*
|
|
Nodal status |
20 (35.7) |
12 (21.4) |
24 (42.9) |
56 (5.2) |
37.6839 |
0.0001*
|
|
Increased vascularity |
278 (58.9) |
141 (29.9) |
53 (11.2) |
472 (43.8) |
7.9112 |
0.0190*
|

For LNs showing SH pattern (121 LNs), 46.3% were seen in WDSCC, 36.4% were seen in MDSCC, and 17.4% were seen in PDSCC. There was statistical significance (
P=0.0500) found between tumor grade and SH pattern in LNs.(
Table 7)
Of 255 LNs showing PCH pattern, 72.2% were in WDSCC cases, 14.1% were in MDSCC cases, and 13.7% were in PDSCC cases. The relationship between tumor grade and PCH pattern was statistically significant (
P=0.0001).(
Table 7)
Of 299 LNs showing CH pattern, 69.6% were in WDSCC cases, 17.1% were in MDSCC cases, and 13.4% were in PDSCC cases. Tumor grade and pattern of reactivity had a statistically significant relationship (
P=0.00001).(
Table 7)
The GCP pattern was seen in 375 LNs, and the UNP was observed in 318 LNs. These patterns showed no significant association with different tumor grades.(
Table 7)
Of all 1,078 LNs that were assessed, 5% showed metastasis. Among these LNs (56 nodes), 42.9% were in PDSCC cases, 35.7% were in WDSCC cases, and 21.4% were in MDSCC cases. This difference was statistically significant (
P=0.0001).(
Table 7)
Of all 1,078 LNs, 472 LNs exhibited increased vascularity. Among these, 58.9% LNs with increased vascularity were WDSCC cases, 29.9% were MDSCC cases, and 11.2% were PDSCC cases. A statistically significant relationship was found between tumor grade and increased vascularity (
P=0.0190).(
Table 7)
Interestingly, patterns of SH, CH, and increased vascularity were found in positive and adjacent nodes more than LP and GCP patterns.(
Table 8)
Table 8
Observation of reactivity patterns in cases of oral squamous cell carcinoma with metastatic nodes
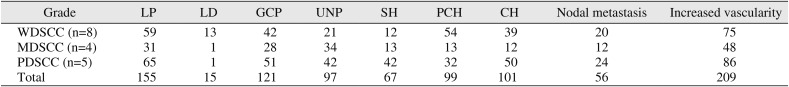
|
Grade |
LP |
LD |
GCP |
UNP |
SH |
PCH |
CH |
Nodal metastasis |
Increased vascularity |
|
WDSCC (n=8) |
59 |
13 |
42 |
21 |
12 |
54 |
39 |
20 |
75 |
|
MDSCC (n=4) |
31 |
1 |
28 |
34 |
13 |
13 |
12 |
12 |
48 |
|
PDSCC (n=5) |
65 |
1 |
51 |
42 |
42 |
32 |
50 |
24 |
86 |
|
Total |
155 |
15 |
121 |
97 |
67 |
99 |
101 |
56 |
209 |

Go to :

IV. Discussion
OSCC ranks among the ten most common cancers of the human body
1. Despite advances in treatment modalities, the survival rate of OSCC remains poor. This could be attributed to locoregional recurrence that occurs secondary to metastasis
1. Because there is an increasing incidence of LN metastasis in the head and neck region, a definite system based on the immune-morphogenic features of LNs is needed to predict prognosis and treatment outcomes
6.
In the present study, 80% of OSCC cases were in male patients. This is in accordance with studies showing that the higher incidence of tobacco intake among males is a key causative agent
367.
Regarding the pattern of the invasive front of tumor cells, large islands were found in 94.4% of WDSCC cases, whereas 52.4% of MDSCC cases showed small islands, and all cases of PDSCC showed individual cells. There was a statistically significant relationship between tumor grade and invasive front (
P=0.0001). As tumor grade increases, tumor cells invade in more undifferentiated patterns as per Broder's classification
6891011.
Studies suggest that lymph vessels and veins are associated with a greater incidence of locoregional LN metastasis in oral cancer, making them important prognostic markers. In the present study, 40% of both WDSCC and PDSCC cases had vascular invasion. This is in contrast to the findings of Anneroth et al.
6, wherein an increased number of cases exhibited invasion with increased grade from WDSCC to PDSCC
6891011. This inconsistent finding is probably due to the borderline invasion in some cases of early stage OSCC
6.
In this study, 71.4% of PDSCC cases were positive for me tastasis. Cells of PD carcinomas lose their cellular adhesion and can migrate further through blood and lymphatics, exhibiting aggressive biological behavior. Metastasis of individual cells is difficult to diagnose by routine histopathology. Studies suggest that LN involvement is an important prognostic factor, and the five-year survival rate is poor in cases with LN involvement
1213.
Saldanha
13 indicated that in the presence of strong cellular immunity, the germinal center is nonreactive with a thick paracortex and LN invasion does not occur. According to Tsakraklides et al.
14, LN histology correlated with grade of primary tumor and survival rate in uterine cancer. They found that WDSCC showed a LP pattern and increased survival rate
14151617181920.
Of the 608 nodes harvested from WDSCC cases in the present study, 41.6% LNs showed a LP pattern in which there is an increase in the number of lymphocytes in the paracortex (T cell zone). A LD pattern was noted in only 1.7% of cases, and most were WD carcinomas. LP usually indicates immune exhaustion, and interference with the cytotoxic activity of T lymphocytes and histiocytes deters their action on metastatic tumor cells to favor metastases. This important observation may aid in understanding vascular invasion, recurrence, and poor survival in some cases of WDSCC.
Tumor invasion and lack of antigen presentation or immune response are also associated with SH. Sinus histiocytes accumulate when there is a need to remove bacteria, debris, or invaded tumor cells during invasion or presentation of an tigenic stimuli
5. In this study, more LNs showed SH in poorly differentiated carcinoma than in well and moderately differentiated carcinoma. Research has indicated that LNs with SH are associated with poor survival
14151617181920.
PCH is also an important reactive pattern exhibited in LNs. Host defense cells stimulated by antigen presentation of follicular dendritic cells proliferate in the paracortical zone. Histologically, this presents as proliferation of lymphocytes in the paracortex with abnormal expansion of the interfollicular zone confined to the capsule. In the present study, 30.3% of LNs without metastasis had PCH in cases of well-differentiated carcinoma. Literature has shown a relationship between PCH with vascularity and favorable prognosis due to high immune reaction in non-metastatic nodes
18.
Another hyperplastic pattern seen in LNs is CH, wherein there is a confluent pattern of T lymphocyte proliferation in the cortex. CH was seen in 34.2% of LNs in cases of WD carcinomas without metastatic LNs. This is in accordance with a study by Tsakraklides et al.
14, showing that CH is associated with UNP and with absence of LN metastases. CH is considered a form of good immunologic response.
LN vascularity can show multiple patterns, including normal peripheral, hilar, or mixed vascularity, reactive response, or vascularity associated with malignancy. The amount of vascularity depends on the tumor type and extent. In cases of metastatic nodes, there is mixed or peripheral vascularity along with loss of architecture in hilar vessels
21. Thus, these changes are an important factor in diagnosing malignancy in a LN
22. In WD carcinomas, 45.7% of LNs had increased vascularity, which could be due to reactive phenomena, especially in LNs without metastasis
18.
As mentioned earlier, not all regional LNs show the same reaction to antigenic stimuli or tumor cells at a given time. Failure of the immune response to clear tumor cells leads to metastasis in regional LNs. Interestingly, of all 1,078 LNs harvested from 50 cases of neck dissection in this study, only 17 cases with 56 harvested LNs showed metastasis (15.2%). LNs were evaluated in all four levels according to different grades of carcinomas. In WDSCC and MDSCC, maximum metastasis was observed in level II LNs, while PSCC cases showed metastasis predominantly in level I LNs. A study by Yamamoto et al.
23 suggested there might be a correlation between aggressive proliferating tumor cells and metastases
2324. However, we suspect that the phenomenon of skip metastasis could be responsible for the findings observed in WDSCC and MDSCC cases. OSCC mostly spreads through lymphatic channels, and LNs act as anatomic barriers that prevent spread. Positive nodes arise from involvement by metastatic cells. The process of metastasis begins with certain molecular changes that cause a LN to be invaded by tumor cells. For this reason, LN status is an important predictor of prognosis. Immune reactions occur by complex interactions between tumor-associated antigens and lymphoid cells that result in pattern formation. Few studies have described the relationship of reactive LNs and tumor prognosis
1325. The morphologic changes of LNs seen with anti-tumor immune reactivity provide an important tool for study. Recent techniques of sentinel LN biopsies are vital for isolating positive nodes. In this study, the patterns associated with malignant changes indicate the beginning of a new phase of cancer. Reactivity patterns such as SH, CH, and increased vascularity were found to be associated with LN metastasis.(
Fig. 2)
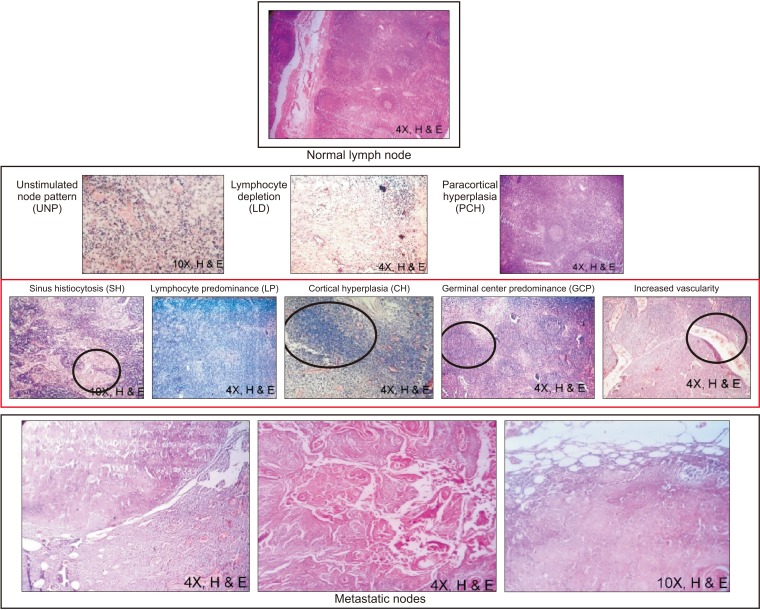 | Fig. 2H&E stained microscopy images (4× or 10×) of reactive patterns most commonly seen in metastatic and adjacent immunoreactive nodes. Selected regions in the pattern (black circles) show greater metastatic potential due to the respective cell predominance.
|
The site and degree of histologic differentiation help to determine tumor cell phenotype and behavior. In the CH pattern, T cells predominate in reaction to metastasis due to tumor-associated antigenic stimuli. In the SH pattern, histiocytes predominate at the margins of metastatic cells due to increased immunoblasts in sinuses, which are prone to invasion. In LP and GCP patterns, immune cells predominate and show tumor response as a result of metastasis
13. With increased vascularity, there is an increased number of feeding vessels at abnormal sites that favor tumor cells instead of the LN.
Other interesting histologic features include the coincidental presence of Warthin's tumor, a tuberculous LN, and extracapsular spread.
The present study demonstrates the importance of analyzing every aspect of LNs. While there may be contradictions and overlapping observations, complete histologic analysis will aid in accurate diagnosis and treatment. In the future, we plan to evaluate LN reactivity patterns immunohistochemically on a larger sample population along with analysis of survival rate.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download