Abstract
Trigeminal nerve injury as a consequence of lower third molar surgery is a notorious complication and may affect the patient in long term. Inferior alveolar nerve (IAN) and lingual nerve (LN) injury result in different degree of neurosensory deficit and also other neurological symptoms. The long term effects may include persistent sensory loss, chronic pain and depression. It is crucial to understand the pathophysiology of the nerve injury from lower third molar surgery. Surgery remains the most promising treatment in moderate-to-severe nerve injuries. There are limitations in the current treatment methods and full recovery is not commonly achievable. It is better to prevent nerve injury than to treat with unpredictable results. Coronectomy has been proved to be effective in reducing IAN injury and carries minimal long-term morbidity. New technologies, like the roles of erythropoietin and stem cell therapy, are being investigated for neuroprotection and neural regeneration. Breakthroughs in basic and translational research are required to improve the clinical outcomes of the current treatment modalities of third molar surgery-related nerve injury.
Lower third molar impaction is common and often leads to pathological conditions like dental caries, pericoronitis, root resorption and cysts that require surgical extraction of the causative tooth12. Neurosensory deficit is a potential permanent complication of lower third molar surgery345. The inferior alveolar nerve (IAN) and lingual nerve (LN) are anatomically lying in close proximity to the surgical site and are at risk to injury when a third molar is removed. IAN deficit affects the cutaneous somatic sensation of the lower lip, while LN deficit affects the sensation of the anterior two-third of the tongue of the ipsilateral side. Chorda tympani, a branch of the facial nerve, runs with the LN that supplies the taste sensation of the same area, is also at risk when the LN is traumatized. The prevalence of lower third molar surgery-related nerve injury was reported to lie within a wide range, that for IAN deficit was reported to be 0.2% to 8.4% and LN deficit was reported to be 0.1% to 22%6. More recent studies with larger sample size, however, reported a much lower prevalence of within 1%, indicating the more factual figure of nerve injury of third molar surgery in the general population47.
It is important to understand the mechanism of the nerve injury to predict the likelihood of recovery as well as to formulate the treatment plan. During lower third molar surgery, the IAN or the LN can be directly or indirectly traumatized by the tooth or by surgical instruments. For IAN, the most likely risk of nerve injury is from the tooth roots' proximity to the nerve, which are reflected by deeper impaction of the third molar, specific radiographic signs in orthopantomogram, or proof of direct contact of the tooth root and the nerve by cone-beam computer tomography or direct visibility of the nerve bundle after extraction89. When direct contact of the root to the nerve exists, the force of the tooth elevation by the surgical instruments may be transmitted to the fragile nerve bundle that causes compression injury. Specific radiographic signs of orthopantomogram like “darkening of the third molar root” may indicate the IAN notches on the third molar root8. In such a scenario even the most experienced surgeons may run into a high risk of IAN injury. Older age was also found to be a risk factor of IAN injury6. It was hypothesized that the bone was not as expandable as the younger individuals, that induce more pressure onto the nerve when the root was elevated. The resultant nerve injury is usually neurapraxia in mild compression, or axonotmesis from the severe compression by the root. Neurotmesis is relatively rare, unless the nerve notches deeply to the tooth root. In contrast, the mode of injury for LN is usually different. The LN lies within the soft tissue medial to the lingual plate of the lower third molar. Distally impacted lower third molars was also identify to be a risk factor of nerve injury since the path of tooth withdrawal is towards disto-lingually, where usually the LN is closest to the periosteum6. There was a debate of whether a lingual flap retractor would protect the LN from direct injury, or the instrument insertion might cause compression neurapraxia10. Researchers who supported the placement of lingual flap retractor argued even if neurapraxia occurred, it was likely to be temporary11. Nonetheless, the position of LN is prone to suffer direct sharp injury if the incision is placed too lingually. When compared to IAN, LN has a higher risk of neurotmesis and may results in a greater need of microsurgical repair12.
IAN and LN are the sensory branches of the trigeminal nerve. It is logical to deduce IAN or LN injury result in total loss (anaesthesia) or at least reduction of mechanoreception and nociception (hypoaesthesia) of the supplying region. Anaesthesia usually implies more severe nerve injury as a result of conduction loss of the supplying nerve, while hypoaesthesia may hint the neural connection is traumatized but at least maintained. There are other symptoms; however, that usually affect the individual in a more significant manner. Hyperaesthesia (i.e., increased sensation from a normal stimulus) and/or dysaesthesia (pain sensation) may be triggered from a stimulation or spontaneously. Severe compression or transection of nerve may initiate neural degeneration and demyelination. It was proved that a nerve impulse arriving at a demyelinated nerve may induce atypical burst of discharges and contributes to the painful symptoms13. When the nerve is partially or totally severed, a neuroma will be formed as an attempt of healing. The outgrowth of axons are the attempts to seek the opposing nerve endings, which may result in a haphazard structure of neural substances and scar tissue. It was shown that neuromas might form a very high sensitivity to mechanical disturbances, which account for the unpleasant sensation as a consequence of the injury14. Taste loss as a collateral damage of the chorda tympani from an LN injury, may be a disturbing symptom of the affected individual. Taste sensation is received by the special sensory component of both the facial nerve (anterior two third of tongue) and the glossopharyngeal nerve, and the olfactory sensation contributes significantly the interpretation of the taste in the higher centre. As a personal observation, it is interesting to note in the Asian population that the effect of taste loss on one side of the anterior two third of tongue appears to impact on the individuals quality of life than people in western countries. It was hypothesized that in general many Asian cultures consider tasting of food to be an important aspect of life. The complication of taste loss from a lower third molar surgery shall not be overlooked.
Since IAN and LN deficit affect sensation, it is important to understand the impact of the affected individual from their perspective. From our study using instruments for patientreported outcome measures, it was found that the perceived general health-related quality of life of the affected individuals was worse than the normal individuals15. Interestingly, of the two components in the measurement, the mental health component was significantly affected when a permanent nerve injury existed while the physical health component was not much affected. It indicates IAN or LN deficit themselves may not affect much the actual physical function of the individual, but it causes a large negative impact on their psychology. We also showed that patients with persistent third molar surgery-induced LN or IAN deficit were having more depression symptoms and were less satisfied in life when compared to normal individuals16. In the same study, it is shocking to note that older patients (over 40 years) had more severe depression symptoms when compared to the younger counterparts when IAN or LN injury occurred16. We hypothesized that older individuals were less able to cope with the negative feeling of the nerve injury. Many patients and surgeons might consider a third molar surgery to be a minor oral surgery without much risk, or did not expect the outcome of the risk. The unhappy patients with nerve injury may take medico-legal action for the compensation when these unpleasant complications occur. In Hong Kong, 42% of the patients who underwent microsurgical nerve repair from third molar surgery-related nerve injury had medico-legal action17. It is therefore important to have sufficient informed consent with the patients preoperatively.
There are many possible assessment tools that are used for the assessment of nerve injury severity18. This section is not intended to describe every single test that is being used. It is important to know how these tests may relate to the clinical condition and to make a proper clinical diagnosis of the nerve injury. It is useful to understand the recovery pattern of spontaneous recovery, and as post-treatment review to assess the treatment outcome. It is even more relevant to use these tests to aid in the clinical judgment of the treatment strategy, especially on the important decision of whether surgical treatment shall be provided. In principle, neurosensory tests can be categorized into subjective and objective assessments. Subjective assessments are often performed through patient interviewed or self-reported symptoms, which are usually reported in visual analogue scale1920. Questionnaires asking specific questions on the symptoms may also be used21. Subjective assessments represent the condition from the patients' perspective and are their genuine feelings. The limitations are of course there is no objective measurements and may not be “scientific” enough. Patient's psychology to the nerve injury may also induce bias to the subjective reporting. Objective neurosensory tests try to quantify the nerve deficit. It is particularly useful to compare with the normal side and for longitudinal changes measurement. Devine et al.18 gave a very detailed listing of the common objective neurosensory tests and their respective implications. However, it is very difficult to compare the nerve injury and treatment outcomes with these many heterogenous neurosensory tests in different studies. The Medical Research Council Scale (MRCS) was developed from peripheral nerve injuries assessment to be a more standardized measurement of sensory recovery22. The term “functional sensory recovery” (FSR) was widely used as a treatment goal, which represent the return of some superficial pain or tactile sensation without over-reaction, with the presence of static two point discrimination of 15 mm or better. The advantages of using FSR are three folds: 1) it helps to quantify the sensory recovery across studies; 2) achieving FSR implies the recovery of the basic protective sensation without unpleasant overreaction; and 3) it reflects in reality the challenge of achieving complete sensory recovery. It is essential to use a standardized assessment like the MRCS to compare different treatment outcomes from different studies to allow improvement of surgical techniques as well as to evaluate the outcomes of newer technologies.
Surgical treatment of third molar surgery-related nerve injury has been the mainstream treatment and the clinical outcomes have been studied for many years. It is proven effective in curing or at least improving symptoms related to the nerve injury23. The mode of injury and anatomical environment are different for IAN and LN injuries, and therefore the treatment rationales are different as well.
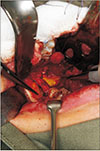
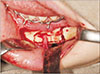
Since LN lies within the soft tissue, when it is severed, the nerve endings retract and undergo degeneration, and often follow by the formation of a neuroma surrounded by scar tissue. Third molar surgery-related LN injuries are mostly caused by a cut by sharp instruments (blade or elevators), or rotary drills, which induce a relatively short defect. Historical cadaveric studies showed LN may lie in close proximity to the lingual plate, and the LN can be traumatized easily when the lingual plate and periosteum are breached24. For milder LN injury where only the superficial nerve structures like the epineurium is damaged, the LN may adhere to the lingual plate periosteum by scar tissue after the healing process25. When injury penetrates deeper or transecting the LN, a neuroma-in-continuity will be formed. The resultant neuromas are usually within 8–15 mm.(Fig. 1) Although milder LN tends to present with milder symptoms, where more severe cases present with worse symptoms accompanies by taste loss, it is sometimes difficult to differentiate the degree of nerve injury based purely on clinical symptoms. Surgical exploration of LN is often the first step of a surgical treatment to identify the status of the nerve by direct visual examination. An external neurolysis can be performed to free the LN from the periosteum that is hold by scar tissue25. However, if a neuroma is present, excision of the neuroma followed by direct suturing will be required to allow reanastomosis of the nerve endings26. In a systematic review conducted by our centre, it was found that complete recovery after surgical treatment of third molar surgery-related LN deficit is rare, with only 25% for external neurolysis and 5.7% for direct suturing12. It is encouraging to note; however, that the majority of patients (around 90%) will have at least some improvement to significant improvement after direct suturing12. Our prospective longitudinal study supplemented that 75% of patients with pre-operative dysaesethesia were pain free after LN direct suturing17. Taste recovery was unpredictable, with 60% have improved and only 10% restored normal taste of the affected region. In the same study, FSR was achieved from 6 months onwards in LN direct suturing17.
A key factor of success for direct suturing of LN is a tension-free repair27.(Fig. 2) After sufficient resection of the neuroma to expose healthy fascicles, the LN may be mobilized from adjacent structure to allow a tension-free repair if the defect is small (usually within 10 mm). When further mobilization is required, the distal end of the LN can be traced antero-inferiorly to where it branches down to supply the submandibular ganglion. The branches can be cut to allow the LN to be mobilized for the neurorrhaphy. When the defect is large, vein graft and Gore-Tex tubing to bridge the defect for neural regeneration have been proposed but did not receive great popularity2829. Autogenous nerve graft costs a donor site defect and is not favourable. Miloro et al.30 and Zuniga31 both published the use of processed allograft for LN repair and showed promising results. Miloro et al.30 reported higher success rate of achieving FSR when compared to direct suturing, which he suggested it was contributed by the sufficient neuroma removal and tensionless repair. Zuniga31 also found 87% improvement after allograft repair, with 100% FSR if the nerve repair was performed within 90 days.
In lower third molar surgery, the two most common aetiologies of IAN injury are 1) compression or laceration of IAN when the third molar root that touches the IAN is elevated; or 2) direct injury by sharp or rotary instruments on the IAN during root elevation or bone removal. The size of nerve injury site is therefore usually very small when compared to other surgical causes like mandibulectomy or pathology-related injury. Another important point is that IAN lies within the inferior alveolar canal, which is a corticated bony canal. When a small injury of IAN occurs, even in more severe form of nerve injury, the traumatized nerve may tend to repair itself within the bony canal, rather than like in LN injury that may form a big neuroma in the process of healing. It is the author's observation that IAN injury from lower third molar surgery rarely presented with dysaesthesia or dramatic hyperaesthesia, which are both important indications of surgical repair of the nerve. It can be hypothesized that the absence of a traumatic neuroma leads to less likelihood of painful symptoms, and the smaller area of injury usually present with mild-to-moderate hypoaesthesia. The need of an IAN repair for these cases is therefore much less indicated. The reported cases of IAN repair as a consequence of third molar surgery-related nerve injury are much fewer than that reported in the LN repair1217. Another consideration is that the technical requirement and surgical time to repair IAN is more when compared to LN repair. IAN repair requires first to expose the course of the nerve by a sagittal split ramus osteotomy. It is followed by mobilization of the nerve to allow tension-free repair. Since the nerve lies within the bony canal, mobilization is difficult, and usually requires lateralization of the whole nerve all the way from the mental foramen.(Fig. 3) It is also difficult to identify the traumatized section of the nerve since it may be repaired within the canal by scar tissue but not an obvious neuroma. After nerve repair, the occlusion has to be re-established and fixed by titanium plates and screws. The access through trans-oral approach can be challenging, and sometimes it may require a trans-cervical approach32. It may be one of the reasons of reported fewer third molar surgery-related IAN repairs when the surgical challenges are weighed against the potential benefits.
The old proverb of “Prevention is better than cure” is most applicable in the issue of nerve injuries from lower third molar surgery. It is not just because preventing nerve injury reduces the suffering of the affected individuals, the fact from the current evidence is that total recovery after any kind of treatment of a severe IAN or LN injury is rare1923. Understanding the various technical risk factors of IAN and LN injuries will help the clinicians to avoid high-risk techniques that poses risks to the nerve. A classic example was the lingual split technique versus buccal approach for lower third molar removal. Lingual split technique using chisel and mallet was a popular technique in some countries because of its efficiency and minimal instrumentation required. However, since many studies found out that lingual split technique carried much higher LN and IAN deficit, the technique has been fading out in our profession633. The application of buccal approach with rotary instrument to remove lower third molars is one of the reasons of reduced nerve injury prevalence in recent publications.
There are some risk factors of nerve injury that cannot be altered. For example, older age was found to be a risk factor for both LN and IAN, but is not an avoidable risk factor6. Several guidelines are available to recommend when shall a lower third molar be removed3234. Guidelines like the NICE (National Institute for Health and Care Excellence) guideline of the United Kingdom are against prophylactic removal of third molars, and there are more debates over this topic in recent years35. With older patients carrying more risk of nerve injury in lower third molar surgery, it is sensible to assess the necessity of removing the third molars on a case-by-case basis, even when absolute indications of removal are not yet present. The author opined that an impacted lower third molar should be considered to be removed early if it is partially erupted or if a periodontal probe may reach to the unerupted tooth. It is safer in terms of the risk of nerve injury to remove the lower third molar at a younger age of the patient.
Coronectomy is a new technique of third molar surgery that removes only the crown of the third molar but leaving the root behind36. The author has been researching on this technique for over 15 years373839404142. It was proved that coronectomy can prevent IAN injury and is safe in long term3739. A very small portion of the retained roots may migrate into the oral cavity and require a second surgery for removal, but it has been migrated away from the IAN and therefore is safe from IAN injury394041. Xenograft may be placed and can reduce the chance of root migration and exposure4344. It is therefore proven a safe alternative of lower third molar surgery where IAN is at risk.
The current treatment outcome of trigeminal nerve repair is limited by the clinical knowledge as well as the understanding of biological neurosensory healing process. Surgical repair of an injured trigeminal nerve is technically demanding, and not many surgeons have a vast amount of cases to improve the clinical skills and knowledge. Well-designed studies on third molar surgery-related trigeminal nerve repair are few, and most studies need to include nerve injuries of different causes to come up with a bigger sample size. There is an urge to conduct large scale prospective clinical studies to answer some very important clinical questions, for example, when is the best timing to perform nerve repair after injury, or how many sutures are ideal for the reanastomosis. The role of alternative treatment, like acupuncture and low level lasers, shall be explored especially in mild-to-moderate nerve injury cases since surgical treatment may potentially cause more harm than benefit12.
Recent advances in neuroscience have brought some hope to improve the outcome of nerve regeneration and repair. Erythropoietin (EPO) or its derivatives were found to carry a neuroprotective effect in spinal cord injury and stroke cases454647. They were also shown to contribute to neuron regeneration in animal studies48. With more evidence and clinical trials, EPO or its derivatives may be used in early or late trigeminal nerve injury as an alternative of surgical treatment, or as an adjunctive therapy when surgical repair is performed.
One of the reasons of unsatisfactory outcome after nerve repair was suggested by the extensive Wallerian degeneration of the nerve that even after successful macroscopic connection of the nerve, the axons were not reconnected49. Stem cell therapy has been a hot topic of study in recent years for its regenerative potential. In the field of regenerative neurosciences, regenerating neurogenic cells from stem cells is an attractive idea to solve many problems. Stem cells sources from bone marrow aspirates, adipose tissue, periodontal ligament and dental pulp have been isolated and successfully differentiated to neural tissues in animal studies505152. Of all the different neural cell types, Schwann cells were believed to play a crucial role in the endogenous repair of nerve and provide pathway for axon regeneration53. Schwann cells differentiated from stem cells have been tested to be incorporated in the surgical site of nerve repair to improve the axonal reconnection and thus clinical outcome54. All these research findings are potential means to breakthrough our current limitations of trigeminal nerve repair. Translational research is urgently needed to bridge the basic sciences and clinical studies to improve patient care in this field.
Third molar surgery-related nerve injury is a potentially problematic complication that causes sensory disturbances, chronic pain and negative psychological impacts on the affected individual. Current treatment modalities may improve the symptoms of the nerve injury but may not achieve complete recovery. Prevention of nerve injury from third molar surgery is important. Coronectomy as an alternative in high IAN risk cases is proved to be effective and safe in long-term. The strategy of managing asymptomatic third molar shall be considered in a case-by-case approach, bearing in mind older age as a factor of nerve injury risk. Basic science and translational research allow breakthrough of the current limitations in clinical practice. The role of new therapeutics and stem cell therapy may bring hope of improving the outcomes of trigeminal nerve injury treatment.
Figures and Tables
 | Fig. 1A. A third molar surgery-related lingual nerve injury case presented with anaesthesia, severe hyperaesthesia and dysaesthesia, showing a neuroma-in-continuity upon nerve exploration. B. The traumatic neuroma of a length of 12 mm was excised. |
Notes
How to cite this article Leung YY. Management and prevention of third molar surgery-related trigeminal nerve injury: time for a rethink. J Korean Assoc Oral Maxillofac Surg 2019;45:233–240. https://doi.org/10.5125/jkaoms.2019.45.5.233
References
1. Haug RH, Abdul-Majid J, Blakey GH, White RP. Evidenced-based decision making: the third molar. Dent Clin North Am. 2009; 53:77–96. ix

2. Suter VGA, Rivola M, Schriber M, Leung YY, Bornstein MM. Risk factors for root resorption of second molars associated with impacted mandibular third molars. Int J Oral Maxillofac Surg. 2019; 48:801–809.

3. Bataineh AB. Sensory nerve impairment following mandibular third molar surgery. J Oral Maxillofac Surg. 2001; 59:1012–1017. discussion 1017.

4. Cheung LK, Leung YY, Chow LK, Wong MC, Chan EK, Fok YH. Incidence of neurosensory deficits and recovery after lower third molar surgery: a prospective clinical study of 4338 cases. Int J Oral Maxillofac Surg. 2010; 39:320–326.

5. Lee CT, Zhang S, Leung YY, Li SK, Tsang CC, Chu CH. Patients' satisfaction and prevalence of complications on surgical extraction of third molar. Patient Prefer Adherence. 2015; 9:257–263.
6. Leung YY, Cheung LK. Risk factors of neurosensory deficits in lower third molar surgery: a literature review of prospective studies. Int J Oral Maxillofac Surg. 2011; 40:1–10.

7. Kim JW, Cha IH, Kim SJ, Kim MR. Which risk factors are associated with neurosensory deficits of inferior alveolar nerve after mandibular third molar extraction? J Oral Maxillofac Surg. 2012; 70:2508–2514.

8. Leung YY, Cheung LK. Correlation of radiographic signs, inferior dental nerve exposure, and deficit in third molar surgery. J Oral Maxillofac Surg. 2011; 69:1873–1879.

9. Ghai S, Choudhury S. Role of panoramic imaging and cone beam CT for assessment of inferior alveolar nerve exposure and subsequent paresthesia following removal of impacted mandibular third molar. J Maxillofac Oral Surg. 2018; 17:242–247.

10. Pichler JW, Beirne OR. Lingual flap retraction and prevention of lingual nerve damage associated with third molar surgery: a systematic review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 91:395–401.

11. Pogrel MA, Goldman KE. Lingual flap retraction for third molar removal. J Oral Maxillofac Surg. 2004; 62:1125–1130.

12. Leung YY, Fung PP, Cheung LK. Treatment modalities of neurosensory deficit after lower third molar surgery: a systematic review. J Oral Maxillofac Surg. 2012; 70:768–778.

13. Burchiel KJ. Abnormal impulse generation in focally demyelinated trigeminal roots. J Neurosurg. 1980; 53:674–683.

14. Meyer RA, Raja SN, Campbell JN, Mackinnon SE, Dellon AL. Neural activity originating from a neuroma in the baboon. Brain Res. 1985; 325:255–260.

15. Leung YY, McGrath C, Cheung LK. Trigeminal neurosensory deficit and patient reported outcome measures: the effect on quality of life. PLoS One. 2013; 8:e77391.

16. Leung YY, Lee TC, Ho SM, Cheung LK. Trigeminal neurosensory deficit and patient reported outcome measures: the effect on life satisfaction and depression symptoms. PLoS One. 2013; 8:e72891.

17. Leung YY, Cheung LK. Longitudinal treatment outcomes of microsurgical treatment of neurosensory deficit after lower third molar surgery: a prospective case series. PLoS One. 2016; 11:e0150149.

18. Devine M, Hirani M, Durham J, Nixdorf DR, Renton T. Identifying criteria for diagnosis of post-traumatic pain and altered sensation of the maxillary and mandibular branches of the trigeminal nerve: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018; 125:526–540.

19. Hillerup S, Stoltze K. Lingual nerve injury II. Observations on sensory recovery after micro-neurosurgical reconstruction. Int J Oral Maxillofac Surg. 2007; 36:1139–1145.
20. Sandstedt P, Sörensen S. Neurosensory disturbances of the trigeminal nerve: a long-term follow-up of traumatic injuries. J Oral Maxillofac Surg. 1995; 53:498–505.
21. Smith JG, Elias LA, Yilmaz Z, Barker S, Shah K, Shah S, et al. The psychosocial and affective burden of posttraumatic neuropathy following injuries to the trigeminal nerve. J Orofac Pain. 2013; 27:293–303.

22. Mackinnon SE. Surgical management of the peripheral nerve gap. Clin Plast Surg. 1989; 16:587–603.

23. Ziccardi VB, Zuniga JR. Nerve injuries after third molar removal. Oral Maxillofac Surg Clin North Am. 2007; 19:105–115. vii

24. Kiesselbach JE, Chamberlain JG. Clinical and anatomic observations on the relationship of the lingual nerve to the mandibular third molar region. J Oral Maxillofac Surg. 1984; 42:565–567.

25. Joshi A, Rood JP. External neurolysis of the lingual nerve. Int J Oral Maxillofac Surg. 2002; 31:40–43.

26. Robinson PP, Loescher AR, Smith KG. A prospective, quantitative study on the clinical outcome of lingual nerve repair. Br J Oral Maxillofac Surg. 2000; 38:255–263.

27. Millesi H. The current state of peripheral nerve surgery in the upper limb. Ann Chir Main. 1984; 3:18–34.

28. Pogrel MA, Maghen A. The use of autogenous vein grafts for inferior alveolar and lingual nerve reconstruction. J Oral Maxillofac Surg. 2001; 59:985–988. discussion 988–93.

29. Pogrel MA, McDonald AR, Kaban LB. Gore-Tex tubing as a conduit for repair of lingual and inferior alveolar nerve continuity defects: a preliminary report. J Oral Maxillofac Surg. 1998; 56:319–321. discussion 321–2.

30. Miloro M, Ruckman P 3rd, Kolokythas A. Lingual nerve repair: to graft or not to graft? J Oral Maxillofac Surg. 2015; 73:1844–1850.

31. Zuniga JR. Sensory outcomes after reconstruction of lingual and inferior alveolar nerve discontinuities using processed nerve allograft--a case series. J Oral Maxillofac Surg. 2015; 73:734–744.

32. LaBanc JP, Van Boven RW. Surgical management of inferior alveolar nerve injuries. Oral Maxillofac Surg Clin North Am. 1992; 4:425–437.

33. Pippi R, Spota A, Santoro M. Prevention of lingual nerve injury in third molar surgery: literature review. J Oral Maxillofac Surg. 2017; 75:890–900.

34. National Institute for Health and Care Excellence (NICE). Guidance on the extraction of wisdom teeth. London: NICE;2000.
36. Leung YY, Cheung LK. Can coronectomy of wisdom teeth reduce the incidence of inferior dental nerve injury? Ann R Australas Coll Dent Surg. 2008; 19:50–51.
37. Leung YY, Cheung LK. Safety of coronectomy versus excision of wisdom teeth: a randomized controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108:821–827.

38. Leung YY, Cheung LK. Coronectomy of the lower third molar is safe within the first 3 years. J Oral Maxillofac Surg. 2012; 70:1515–1522.

39. Leung YY, Cheung LK. Long-term morbidities of coronectomy on lower third molar. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016; 121:5–11.

40. Leung YY, Cheung KY. Root migration pattern after third molar coronectomy: a long-term analysis. Int J Oral Maxillofac Surg. 2018; 47:802–808.

41. Yeung AWK, Wong NSM, Bornstein MM, Leung YY. Three-dimensional radiographic evaluation of root migration patterns 4-8.5 years after lower third molar coronectomy: a cone beam computed tomography study. Int J Oral Maxillofac Surg. 2018; 47:1145–1152.

42. Yeung AWK, Wong NSM, Leung YY. Are coronectomy studies being cited? A bibliometric study. J Investig Clin Dent. 2019; 10:e12366.

43. Leung YY. Coronectomy of lower third molars with and without guided bony regeneration: a pilot study. Br J Oral Maxillofac Surg. 2016; 54:155–159.

44. Leung YY. Guided bone regeneration to reduce root migration after coronectomy of lower third molar: a randomized clinical trial. Clin Oral Investig. 2019; 23:1595–1604.

45. Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000; 97:10526–10531.

46. Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002; 8:495–505.

47. Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev. 2004; 27:113–120.

48. Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001; 21:9733–9743.

50. Walsh S, Midha R. Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurg Focus. 2009; 26:E2.

51. Techawattanawisal W, Nakahama K, Komaki M, Abe M, Takagi Y, Morita I. Isolation of multipotent stem cells from adult rat periodontal ligament by neurosphere-forming culture system. Biochem Biophys Res Commun. 2007; 357:917–923.

52. Pereira LV, Bento RF, Cruz DB, Marchi C, Salomone R, Oiticicca J, et al. Stem cells from human exfoliated deciduous teeth (SHED) differentiate in vivo and promote facial nerve regeneration. Cell Transplant. 2019; 28:55–64.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download