CASE REPORT
An 82-year-old man (64.5 kg, 175 cm) diagnosed with falcine meningioma was admitted for craniotomy. The patient presented left-sided weakness. Comorbidities included atrial fibrillation and Parkinson's disease. He had undergone prostate surgery under spinal anesthesia 1 year previously. Preoperative thoracic radiographs revealed several small nodular opacities in the upper lobe of the right lung. Low-dose thoracic computed tomography (CT) was performed to further evaluate the possibility of pulmonary tuberculosis, which revealed fibro-atelectatic changes, bronchiectasis, calcifications, and small nodules, considered to be sequelae to resolved pulmonary tuberculosis. The patient presented no respiratory symptoms, and pulse oximetry readings were 100% in room air. He was a non-smoker. Tracheobronchomegaly was not detected on the preoperative thoracic radiograph as the image was not clear enough to distinguish the trachea. Therefore, the anesthesia plan was to manage the patient as if he had chronic obstructive pulmonary disease, in terms of his pulmonary condition.
After denitrogenation with 100% oxygen, anesthesia was induced via intravenous propofol and remifentanil infusion. After confirming the loss of consciousness and following sufficient manual ventilation, 50 mg of rocuronium was injected prior to intubation. A reinforced tracheal tube with an inner diameter of 7.5 mm was inserted and fixed at 23 cm from the incisors. An arterial catheter was placed in the left radial artery and central venous catheter in the right subclavian vein. Total intravenous anesthesia was maintained using a target-controlled infusion system.
After the completion of central venous catheterization, tidal volume was considered insufficient and breathing sounds were reduced on auscultation in both lung fields. The ventilator circuit was checked for respiratory leakage, and no leak was found. The patient was reintubated with a new tracheal tube in case of tube malfunction; however, lack of airway control consistent with air leakage persisted. The extubated tube was examined, and no malfunction was identified.
At this stage, an airway deformity was suspected, and thoracic CT images were reviewed in the operating room. The patient's tracheal diameter appeared larger than normal. One anesthesiologist suggested MKS based on his experience of airway management in a patient with MKS in the past [
2]. Because of possible damage to the tracheal wall due to additional expansion of the tracheal tube cuff, the oropharyngeal cavity was sealed with wet gauze packing, which allowed us to achieve an adequate tidal volume. The total operation time was 8 hours. Postoperatively, the patient was sedated with a benzodiazepine and transferred to the intensive care unit without tracheal extubation.
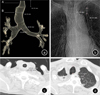
The transverse diameter of the patient's trachea was measured 2 cm above the aortic arch on a chest scanogram (similar to a simple chest X-ray) as 25.4 mm (
Fig. 1B). Transverse and sagittal diameters of the trachea at the same level were 26.8 and 32.0 mm, respectively, on the CT images (
Fig. 1C, 1D). These measurements were consistent with the diagnostic criteria for MKS. After surgery, a three-dimensional (3D) reconstruction of the preoperative chest CT was created, which confirmed the abnormal anatomical morphology of the trachea (
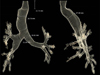
Fig. 1A and
2). The patient was extubated on the same day as surgery. Eight days post-surgery, he was discharged without acute complications.
DISCUSSION
MKS is a rare medical condition. Since its first description in 1932, approximately 130 cases have been reported in the English literature [
1]. MKS is more common in men. There does not seem to be any correlation between the age at diagnosis and tracheal diameter. MKS is associated with bronchiectasis, tracheal diverticulosis, and tracheobronchial dyskinesia. Clinical signs usually include nonspecific respiratory symptoms such as cough, dyspnea, and recurrent airway infection [
1].
MKS can be diagnosed based on tracheal or bronchial diameter measurements. Previous studies have reported standard values for the normal tracheal and mainstem bronchial diameters based on radiological examination [
3], bronchography [
4], and thoracic CT [
5]. MKS is diagnosed based on deviation from these normal values [
46].
MKS has rarely been reported in the anesthesia field. Only seven cases have been reported on airway management during general anesthesia [
2789101112]. The most important aspect of managing patients with MKS during general anesthesia is airway maintenance. A standard-sized tracheal tube may allow peri-tubal air leakage in the dilated trachea, which can lead to inappropriate tidal volumes and/or pulmonary aspiration. Therefore, close preoperative evaluation and careful pre-anesthetic planning are required for these patients. Among the seven reported cases, two introduced successful one-lung ventilation during pulmonary surgery [
812] and the other five described airway management via intubation with a single lumen tracheal tube. In three of these five cases, large-sized tubes were used and/or the cuff was placed in the subglottic area [
2811]. In one case, intra-oral gauze packing was used to prevent air leakage [
10]. In another, laryngeal mask airway (LMA) was used for airway maintenance [
9].
Thus far, there is limited consensus on airway management of patients with MKS during general anesthesia. One author in our team had previous experience of managing the airway of a patient with MKS, involving subglottic placement of the cuff of a large-sized tracheal tube [
2]. In that case, the patient did not experience postoperative complications such as tracheal stenosis due to the tube cuff [
2], despite the recommendation by some physicians of using a cuffless tube with oral packing to avoid this possible complication [
13]. Some physicians have also recommended creating an airtight seal using a large-sized tracheal tube and/or subglottic placement of tube cuff to prevent pulmonary aspiration [
27]. However, in the present case, aspiration of the gastric content did not occur despite the lack of these precautions.
In our case, the tracheal diameter was measured (using CT images) in the operating room, and it was determined that there was no suitable point for tube cuff placement other than the subglottic area. Although previous cases have successful created an airtight seal using a large-sized tracheal tube or with the tube cuff placed in the subglottic area [
2711], the use of a large-sized tube and/or excessive inflation of the tube cuff was thought to increase the risk of mucosal injury to the trachea; therefore, it was not used in this case. A previous report has demonstrated severe tracheal stenosis following the use of a tracheal tube, even with normal cuff pressure, in a patient with MKS [
14]. Using LMA was also difficult in our case as the patient was undergoing neurosurgery, with a prolonged operation time predicted. Therefore, airway stability may have been uncertain, and it would not have been easy to handle the LMA if it had become displaced. Therefore, we used a combination of oral packing and a tracheal tube with a standard inner diameter (7.5 mm for a man). This allowed us to maintain the essential tidal volume; however, the risk of pulmonary aspiration remained. We performed gastric evacuation prior to gauze packing in an attempt to decrease this risk. Fortunately, the patient did not develop any symptoms of aspiration. Therefore, anesthesiologists involved in dental surgeries should consider using a large-sized tracheal tube with excessive cuff inflation or oral gauze packing as the use of supraglottic airway access, such as LMA, is not possible.
To date, few reports, including ours, have presented 3D CT reconstructions of the trachea and mainstem bronchi in patients with MKS [
21115], and none of them showed dilation of the trachea in the subglottic area. Further investigation using 3D reconstructed images of the trachea are needed to investigate whether this morphology is typical in MKS, as the subglottic placement of the tracheal tube cuff may be unavoidable in some cases. Furthermore, the diameter of the trachea and main bronchi can be measured more precisely with 3D imaging because the measurement can be performed perpendicular to the longitudinal axis. The diagnostic criteria for MKS may be modified in the future based on more accurate 3D imaging measurements.
In the above previously published cases, MKS was diagnosed preoperatively; therefore, anesthesiologists could prepare a management plan [
2789101112]. However, some patients may be asymptomatic, and MKS may go undetected prior to induction of anesthesia, as in the present report. Precise measurement of the tracheobronchial diameter based on thoracic radiographs can be difficult due to haziness of the image, as was seen in our case. Preoperative thoracic CT scans are not routinely acquired, and airway dilatation can be easily overlooked if the patient has not previously had any airway related symptoms.
MKS is a rare disease that may be easily overlooked and can be fatal if not managed appropriately during general anesthesia. Therefore, anesthesiologists should evaluate patients carefully and set anesthesia plans before surgery. If an anesthesiologist encounters preoperative radiologic findings of bronchiectasis combined with an unexplained air leak after tracheal intubation and cuff inflation, MKS should be suspected. In terms of anesthesia for oral and maxillofacial surgeries, anesthesiologists usually perform nasotracheal intubation using the nasal Ring-Adair-Elwyn (RAE) tubes for general anesthesia. For some patients requiring dental treatment, it is not possible to perform oro-tracheal intubation. However, there is a high risk of epistaxis during nasotracheal intubation. Repeated nasotracheal intubation may not be easy if an airway problem similar to the event described here is encountered during anesthesia. Therefore, we believe that our description of airway management in a patient with MKS provides a helpful and informative resource for clinicians, including anesthesiologists, particularly those working in the field of dental surgery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download