1. Fikree A, Aktar R, Morris JK, Grahame R, Knowles CH, Aziz Q. The association between Ehlers-Danlos syndrome-hypermobility type and gastrointestinal symptoms in university students: a cross-sectional study. Neurogastroenterol Motil. 2017; 29:e12942.

2. Murray B, Yashar BM, Uhlmann WR, Clauw DJ, Petty EM. Ehlers-Danlos syndrome, hypermobility type: A characterization of the patients' lived experience. Am J Med Genet A. 2013; 161A:2981–2988. PMID:
24254846.

3. Schubart JR, Schaefer E, Hakim AJ, Francomano CA, Bascom R. Use of cluster analysis to delineate symptom profiles in Ehlers-Danlos syndrome patient population. J Pain Symptom Manage. 2019; 58:427–436. PMID:
31153935.
4. Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A. A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet. 2017; 175:148–157. PMID:
28145606.

5. Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017; 175:8–26. PMID:
28306229.
6. Chopra P, Tinkle B, Hamonet C, Brock I, Gompel A, Bulbena A, et al. Pain management in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017; 175:212–219. PMID:
28186390.

7. Voermans NC, Knoop H, Bleijenberg G, van Engelen BG. Pain in ehlers-danlos syndrome is common, severe, and associated with functional impairment. J Pain Symptom Manage. 2010; 40:370–378. PMID:
20579833.

8. Schubart JR, Schilling A, Schaefer E, Bascom R, Francomano C. Use of prescription opioid and other drugs among a cohort of persons with Ehlers-Danlos syndrome: A retrospective study. Am J Med Genet A. 2019; 179:397–403. PMID:
30624009.

9. Arendt-Nielsen L, Kaalund S, Bjerring P, Hogsaa B. Insufficient effect of local analgesics in Ehlers Danlos type III patients (connective tissue disorder). Acta Anaesthesiol Scand. 1990; 34:358–361. PMID:
2389651.

10. Arendt-Nielsen L, Kaalund S, Hogsaa B, Bjerring P, Grevy C. The response to local anaesthetics (EMLA-cream) as a clinical test to diagnose between hypermobility and Ehlers Danlos type III syndrome. Scand J Rheumatol. 1991; 20:190–195. PMID:
2068541.
11. Hakim AJ, Grahame R, Norris P, Hopper C. Local anaesthetic failure in joint hypermobility syndrome. J R Soc Med. 2005; 98:84–85. PMID:
15684369.

14. Vreeland DL, Reader A, Beck M, Meyers W, Weaver J. An evaluation of volumes and concentrations of lidocaine in human inferior alveolar nerve block. J Endod. 1989; 15:6–12. PMID:
2607268.

15. Nist RA, Reader A, Beck M, Meyers WJ. An evaluation of the incisive nerve block and combination inferior alveolar and incisive nerve blocks in mandibular anesthesia. J Endod. 1992; 18:455–459. PMID:
9796516.

16. Ridenour S, Reader A, Beck M, Weaver J. Anesthetic efficacy of a combination of hyaluronidase and lidocaine with epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2001; 48:9–15. PMID:
11495405.
17. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377–381. PMID:
18929686.

18. Marhofer D, Karmakar MK, Marhofer P, Kettner SC, Weber M, Zeitlinger M. Does circumferential spread of local anaesthetic improve the success of peripheral nerve block? Br J Anaesth. 2014; 113:177–185. PMID:
24574507.

19. St George G, Morgan A, Meechan J, Moles DR, Needleman I, Ng YL, et al. Injectable local anaesthetic agents for dental anaesthesia. Cochrane Database Syst Rev. 2018; 7:CD006487. PMID:
29990391.

20. Trescot AM. Local anesthetic "resistance". Pain Physician. 2003; 6:291–293. PMID:
16880874.

21. Badr N, Aps J. Efficacy of dental local anesthetics: A review. J Dent Anesth Pain Med. 2018; 18:319–332. PMID:
30637342.

22. Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD. The role of voltage-gated sodium channels in pain signaling. Physiol Rev. 2019; 99:1079–1151. PMID:
30672368.

23. Fang P, Tang PF, Xu RA, Zheng X, Wen J, Bao SS, et al. Functional assessment of CYP3A4 allelic variants on lidocaine metabolism in vitro. Drug Des Devel Ther. 2017; 11:3503–3510.
24. Oliver DW, Balan KK, Burrows NP, Hall PN. Dispersal of radioisotope labelled solution following deep dermal injection in Ehlers-Danlos syndrome. Br J Plast Surg. 2000; 53:308–312. PMID:
10876255.

25. Aktar R, Peiris M, Fikree A, Cibert-Goton V, Walmsley M, Tough IR, et al. The extracellular matrix glycoprotein tenascin-X regulates peripheral sensory and motor neurones. J Physiol. 2018; 596:4237–4251. PMID:
29917237.

26. Aktar R, Peiris M, Fikree A, Eaton S, Kritas S, Kentish SJ, et al. A novel role for the extracellular matrix glycoprotein-Tenascin-X in gastric function. J Physiol. 2019; 597:1503–1515. PMID:
30605228.

27. Vieira WA, Paranhos LR, Cericato GO, Franco A, Ribeiro MAG. Is mepivacaine as effective as lidocaine during inferior alveolar nerve blocks in patients with symptomatic irreversible pulpitis? A systematic review and meta-analysis. Int Endod J. 2018; 51:1104–1117. PMID:
29577321.

28. Sampaio RM, Carnaval TG, Lanfredi CB, Horliana AC, Rocha RG, Tortamano IP. Comparison of the anesthetic efficacy between bupivacaine and lidocaine in patients with irreversible pulpitis of mandibular molar. J Endod. 2012; 38:594–597. PMID:
22515885.

29. Su N, Wang H, Zhang S, Liao S, Yang S, Huang Y. Efficacy and safety of bupivacaine versus lidocaine in dental treatments: a meta-analysis of randomised controlled trials. Int Dent J. 2014; 64:34–45. PMID:
24117122.

30. Visconti RP, Tortamano IP, Buscariolo IA. Comparison of the anesthetic efficacy of mepivacaine and lidocaine in patients with irreversible pulpitis: a double-blind randomized clinical trial. J Endod. 2016; 42:1314–1319. PMID:
27475099.
31. Seneviratne SL, Maitland A, Afrin L. Mast cell disorders in Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2017; 175:226–236. PMID:
28261938.

32. Magadmi R, Meszaros J, Damanhouri ZA, Seward EP. Secretion of mast cell inflammatory mediators is enhanced by CADM1-dependent adhesion to sensory neurons. Front Cell Neurosci. 2019; 13:262. PMID:
31275114.

33. Castori M. Pain in Ehlers-Danlos syndromes: manifestations, therapeutic strategies and future perspectives. Expert Opin Orphan Drugs. 2016; 4:1145–1158.

34. Fossataro C, Bucchioni G, D'Agata F, Bruno V, Morese R, Krystkowiak P, et al. Anxiety-dependent modulation of motor responses to pain expectancy. Soc Cogn Affect Neurosci. 2018; 13:321–330. PMID:
29325145.

35. Drasner K. Katzung BG, editor. Local Anesthetics. In: Basic & Clinical Pharmacology. 14th ed. New York: McGraw-Hill Education;2017.
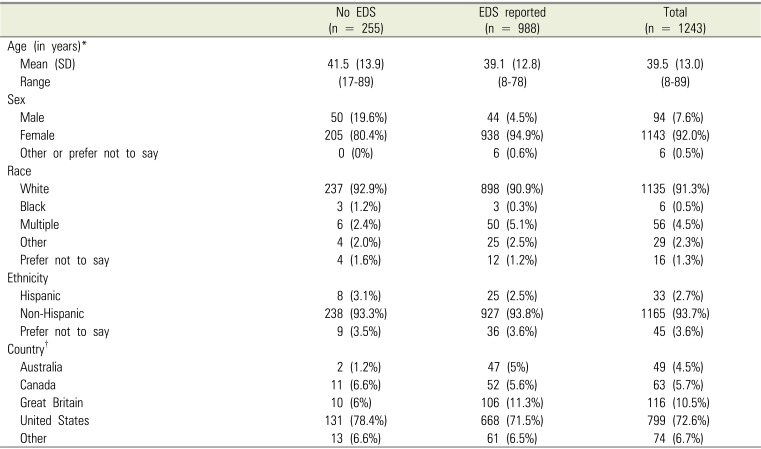
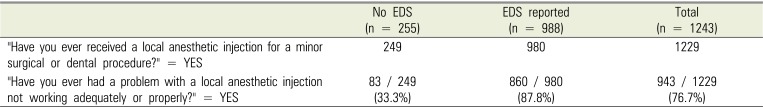
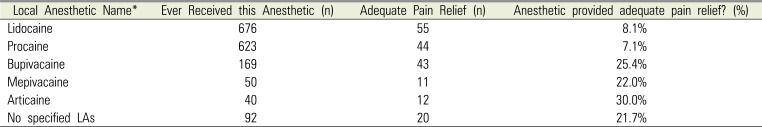
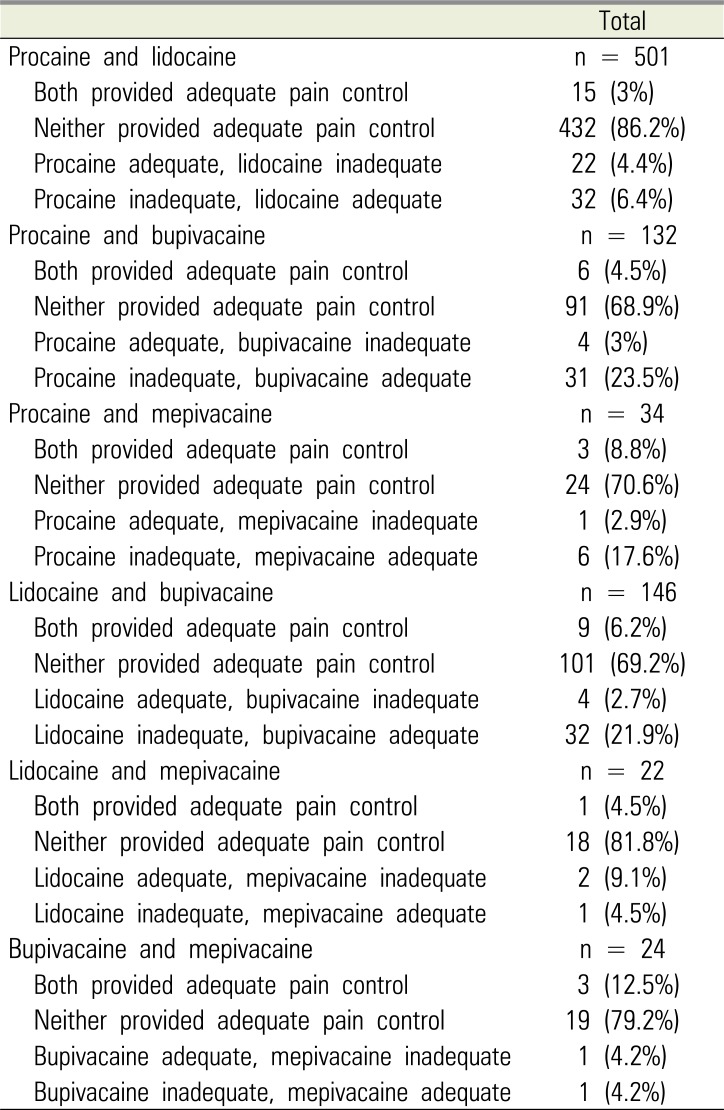




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download