RESULTS
Subjects' characteristics and clinical manifestations
During the study period, 1,017 patients with pathologically confirmed primary tumors that originated from the central nervous system were treated at our center. Among them, 314 had glial brain tumors; 155, glioblastoma; 46, oligodendroglioma; 31, PA; 2, pilomyxoid astrocytoma; 24, ependymoma; 15, ganglioglioma; 13, diffuse astrocytoma; 12, anaplastic astrocytoma; 9, oligoastrocytoma; and 7, gliosarcoma. The ratio of PA to the total number of primary brain tumors was higher in the patients aged <20 years [20.8% (20/96)] than in those aged ≥20 years [1.2% (11/921);
p<0.001]. The ratios of the respective disease groups by patient age are shown in
Fig. 1A.
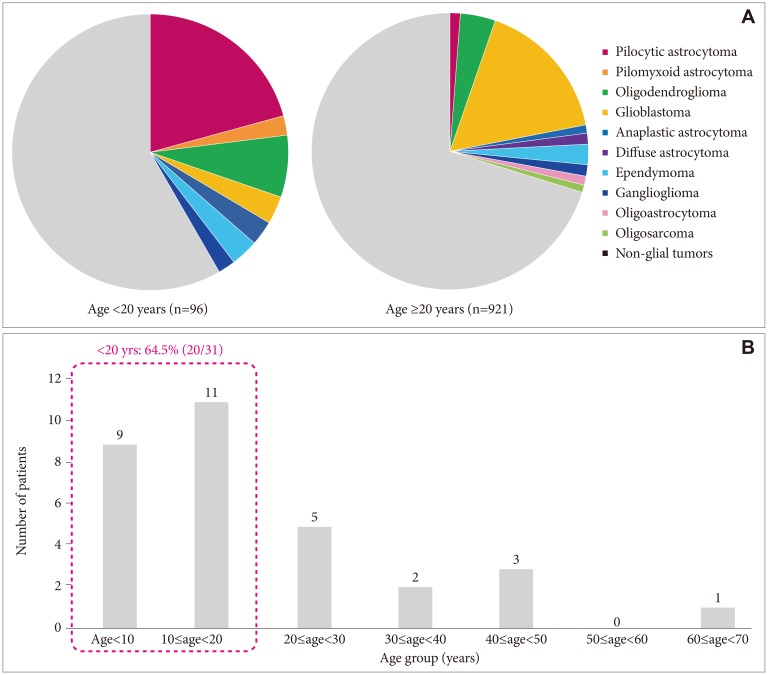 | Fig. 1The ratio of glial tumors between age groups in a single medical center between1988 and 2018. A: A total of 1,017 patients had pathologically confirmed primary brain tumors; of them, 314 had glial tumors. Pilocytic astrocytoma occurred more frequently in patients aged <20 years (20.8%) than in those aged ≥20 years (1.2%; p<0.001). B: The ratio of pilocytic astrocytoma among the patients aged <20 years was 64.5%.
|
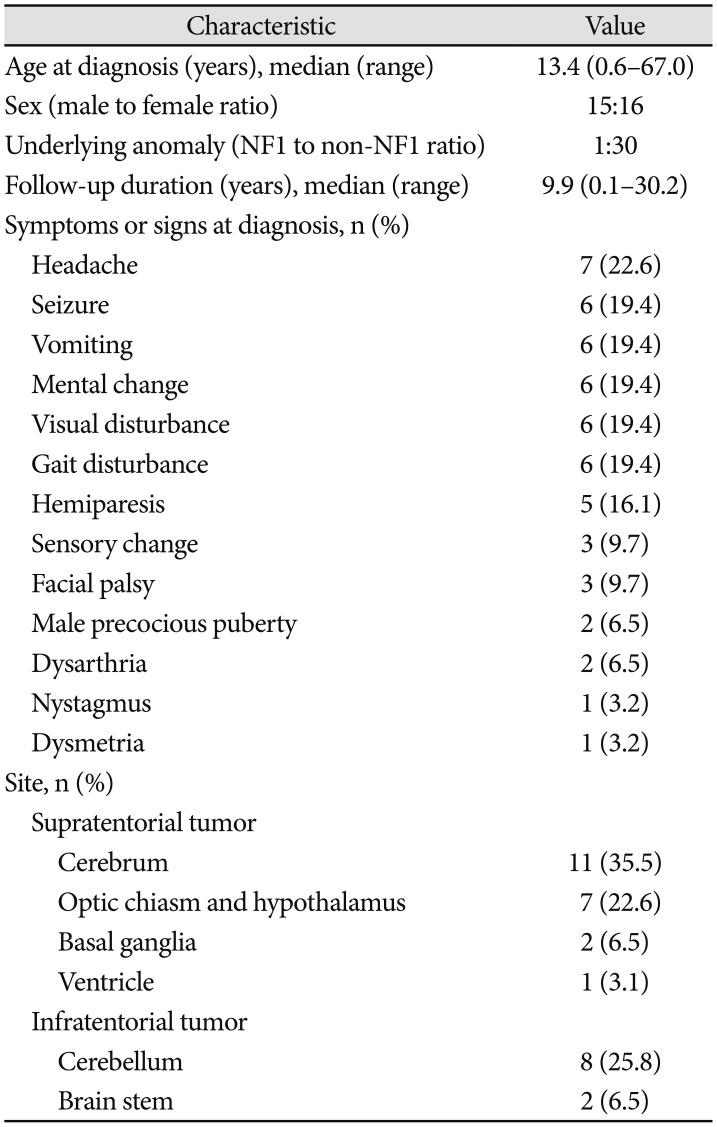
Thirty-one subjects had PA (male-to-female ratio, 15:16). Among them, one had neurofibromatosis 1 confirmed by genetic testing. The median age at diagnosis was 13.4 years (range, 0.6–67.0 years). The median follow-up duration was 9.9 years (range, 0.1–30.2 years). The PA distribution by age is shown in
Fig. 1B. The patients with PA had symptoms or signs that included headache (7), seizure (6), vomiting (6), mental change (6), visual disturbance (6), gait disturbance (6) or hemiparesis (5). The tumor sites were the cerebrum (11), cerebellum (8), optic chiasm and hypothalamus (7), brain stem (2), basal ganglia (2), and ventricle (1). The baseline characteristics of the subjects with PA are summarized in
Table 1.
Table 1
Baseline characteristics of patients with pilocytic astrocytoma treated at a single center

Treatment modalities
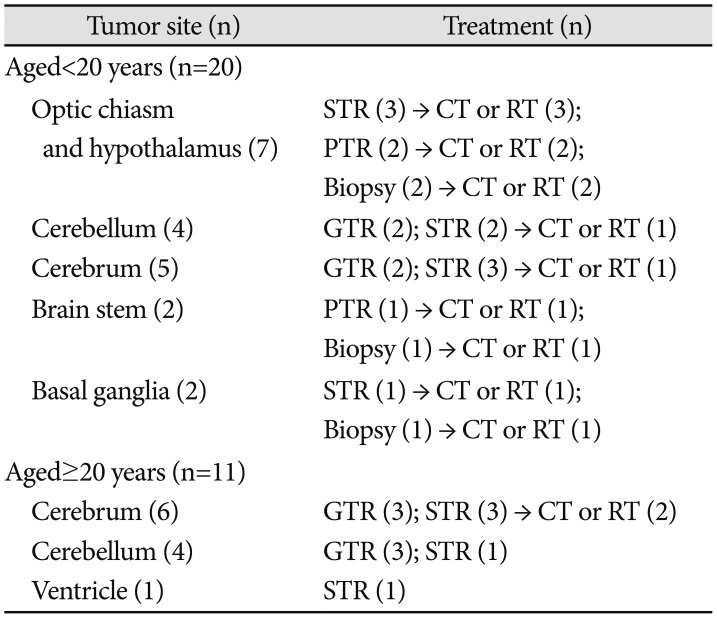
Among the 31 patients with PA, 10 underwent GTR; 14, STR; 3, PTR; and 4, biopsy. In the non-GTR group (n=21), additional immediate chemotherapy and/or radiotherapy were administered to 15 patients (Add-Tx group): chemotherapy was administered to 10 patients and radiotherapy was administered to 7 patients (2 patients received both). In the patients aged <20 years, tumors were more likely to be located in sites where GTR could not be performed, including the optic chiasm and hypothalamus (7), brain stem (2), and basal ganglia (2). The respective treatment modalities for the patients with PA are described in
Table 2. The chemotherapy regimens included: CCG9952A (carboplatin and vincristine) (7), Ghim's protocol [
5] (vinblastine, etoposide, 5-fluorouracil, and cyclophosphamide) (1), and POG9031 (cisplatin and etoposide) (2). The median radiotherapy dose was 5,400 cGy (range, 4,500–6,000 cGy).
Table 2
Treatment modalities for patients with pilocytic astrocytoma treated at a single center

Survival rate
In the total PA group, the 10-year DSS, OS, and PFS were 92.6% (95% CI, 82.6–100), 88.4% (95% CI, 66.4–98.6), and 52.8% (95% CI, 32.0–73.6), respectively (
Fig. 2). Three patients died of causes other than brain tumor progression, 1 patient died of infection and septic shock at 13.9 years after the diagnosis of PA, 1 patient died of a psychological problem and suicide at 10.2 years after diagnosis, and 1 patient died of an unknown cause of death at 3.6 years after diagnosis. No statistically significant difference in 10-year DSS was found among the GTR (100%), STR (92.9%; 95% CI, 79.4–100), PTR (100%), and biopsy (75%; 95% CI, 32.5–100,
p=0.120) (
Fig. 3A) groups. However, a significant statistical difference in 10-year PFS was found among the GTR (100%), STR (43.7%; 95% CI, 15.5–71.9), PTR (0%), and biopsy groups (0%;
p<0.001) (
Fig. 3B). No statistically significant difference in 10-year DSS was found between the GTR (100%) and non-GTR (89.7%; 95% CI, 76.2–100,
p=0.374) (
Fig. 3C) groups. However, a statistically significant difference in 10-year PFS was found between the GTR (100%) and non-GTR (30.7%, 95% CI, 8.6–52.8;
p=0.012) (
Fig. 3D) groups. No statistically significant difference in 10-year DSS was found between the <20-year (94.7%; 95% CI, 84.7–100) and ≥20-year (88.9%; 95% CI, 68.3–100;
p=0.634) age groups. No statistically significant difference in 10-year PFS was found between the <20-year (37.9%; 95% CI, 12.2–63.6) and ≥20-year age group (65.6%; 95% CI, 33.5–97.7;
p=0.432). In the non-GTR group, no statistically significant difference in 10-year DSS was found between the Add-Tx (92.9%; 95% CI, 79.4–100) and non-Add-Tx (83.3%; 95% CI, 53.5–100;
p=0.577) groups. No statistically significant difference in 10-year PFS was found between the Add-Tx (28.9%; 95% CI, 1.7–56.1) and non-Add-Tx (33.3%; 95% CI, 0–70.9;
p=0.706) groups.
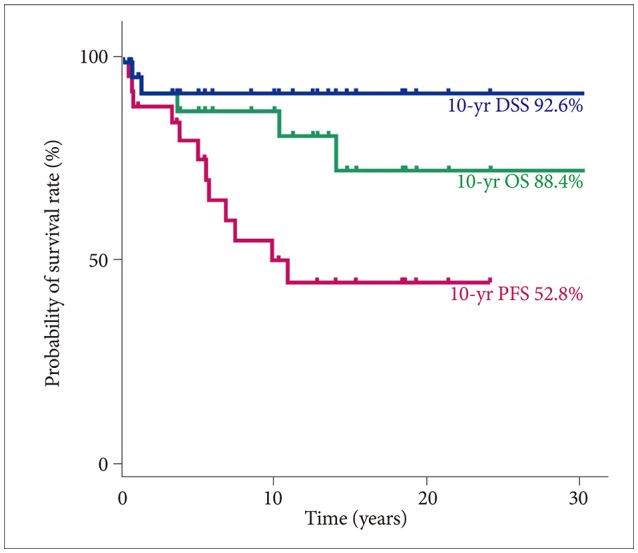 | Fig. 2The survival rate of the patients with pilocytic astrocytoma in a single center. The 10-year DSS, OS, and PFS were 92.6% (95% CI, 82.6–100), 88.4% (95% CI, 66.4–98.6), and 52.8% (95% CI, 32.0–73.6). DSS, disease-specific survival; OS, overall survival; PFS, progression-free survival; CI, confidence interval.
|
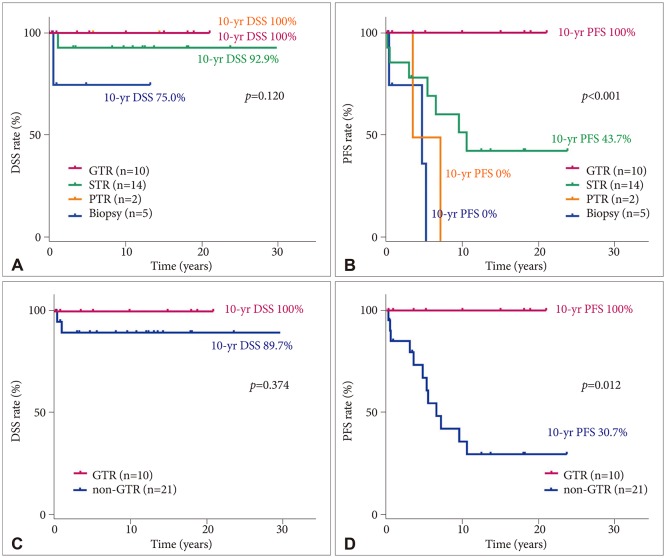 | Fig. 3Survival rates of the patients with pilocytic astrocytoma according to resection success. No statistically significant difference in 10-year DSS was found between the GTR and non-GTR groups (A and C). However, a statistically significant difference in 10-year PFS was observed between the GTR and non-GTR groups (B and D). DSS, disease-specific survival; PFS, progression-free survival; GTR, gross total resection; STR, subtotal tumor resection; PTR, partial tumor resection.
|
Sequelae and complications
The survivors had neurological complications, including seizure (13), headache (4), dysarthria (4), ataxia (4), paralysis (3), fine motor failure (2), dizziness (1), and facial palsy (1). The survivors also had psychiatric complications, including alcohol dependence (3), nervous personality (2), isolation (1), major depressive disorder (1), self-harm (1), suicide (1), somatizing syndrome (1), attention deficit hyperactivity disorder (1), hallucination (1), bulimia nervosa (1), inappropriate behavior (1), and violent behavior (1). They also had endocrine problems, including precocious puberty (4), growth hormone deficiency (4), panhypopituitarism (3), and obesity (1).
Go to :

DISCUSSION
PA is the most common brain tumor with a low-grade histology in the first 2 decades of life [
6]. PAs can be found along the neuraxis, including the optic chiasm, hypothalamus, cerebral hemisphere, and brain stem; 80% of cases are found in the cerebellum [
7]. In our study, PA was also the most common primary brain tumor in patients aged <20 years. However, optic chiasmatic-hypothalamic and brain stem tumors were relatively more frequent in the patients aged <20 years in our study.
Surgical resection is the standard initial approach to managing PA, and GTR is desirable. PA is generally a benign tumor that features excellent outcomes with GTR [
8]. If removal is complete, no further treatment is necessary. However, a PA that arises in the optic pathway, brain stem, and hypothalamus is not usually amenable to GTR. Some factors, including PTR, tumor location, or invasion of the surrounding structures, are associated with a worse prognosis [
8]. In a prospective nonrandomized study of childhood low-grade glioma (LGG) performed by The Children's Cancer Group and Pediatric Oncology Group, the 5-year PFS was 90% with GTR and 45–65% with any volume of residual tumor (
p<0.001) [
9]. Tumor site was also associated with PFS. Chiasmatic-hypothalamic or midline tumors, for which GTR is difficult to perform, showed lower PFS than cerebellar or cerebral tumors [
9].
If resection is incomplete, the need for immediate additional therapy such as chemotherapy or radiotherapy remains controversial among clinicians [
10]. Some evidence indicates that additional radiotherapy may prolong PFS but has little impact on OS [
10]. In the European Organization for Research and Treatment of Cancer study of 22,845 patients, 311 patients with LGG aged ≥16 years were randomized to receive postoperative observation or immediate radiotherapy [
11]. The irradiated patients showed a significantly improved 5-year PFS, but no significant difference in 5-year OS was observed between the 2 arms [
11]. Thus, the authors recommend additional treatment only after re-progression of the tumor and on the basis of the premise that OS will be unaffected regardless of whether PFS significantly improves [
67].
In fact, radiotherapy is generally reserved for cases in which multiple chemotherapy treatments have failed in unresectable symptomatic tumors because of its toxicity. To minimize the risk of late sequelae of brain radiation exposure, the use of chemotherapy to delay radiation exposure in young children has become widespread in pediatric cases. In a recent study that analyzed the efficacy of CV (vincristine and carboplatin) in 113 children with LGG, an overall response to chemotherapy was observed in 92% of the children, while the median time to progression was 22.5 months in 42% of the children [
12]. On the basis of the COG A9952 results, the use of either TPCV [carboplatin, procarbazine, CCNU (lomustine), and vincristine] or CV provides adequate tumor control that allows a radiotherapy delay [
13].
In a single-center study in the Korean capital area that included 91 pediatric patients with PA, the 10-year PFS was higher in those who received postoperative additional treatment, including radiotherapy or chemotherapy, than in those who were only observed [
3]. However, no statistically significant difference in 10-year PFS was found between the Add-Tx and non-Add-Tx groups in our study. We suggest that this is because of the retrospective single-center design of our study with fewer cases. On the other hand, no significant difference in 10-year DSS was observed between the GTR and non-GTR groups in our study. Thus, we suggest that immediate additional treatment such as radiotherapy or chemotherapy would play a role in the treatment of our patients. In a single-center study in the Korean Honam area that included approximately 39 patients with PA, tumor progression in adult patients with PA followed a more aggressive clinical course than that in pediatric patients with PA [
4]. However, no statistically significant difference in 10-year DSS or PFS was found between the <20- and ≥20-years age groups in our study, possibly because the tumors in the patients aged <20 years in our study were relatively more commonly located in areas where surgery is not likely to completely remove them (optic chiasmatic-hypothalamic or brain stem tumors).
The Epidemiology and End Results database reported on the long-term outcomes of 4,040 pediatric patients with LGG [
14]. The 20-year OS was 87%, and the 20-year cumulative incidence of death due to LGG glioma was 12%. Prognostic factors included patient age, tumor histology and grade, primary tumor site, radiation dose, and degree of surgical resection on univariate analysis [
14]. In the multivariate analysis, the greatest mortality risk was associated with the use of radiation [
14]. Thus, the strategies for pediatric LGG should aim for disease control with emphasis on minimizing long-term toxicities associated with treatment [
14]. Neurocognitive and endocrine dysfunctions are considerable factors that could be related with tumor progression or treatment-related toxicity [
15]. Our study also showed several late sequelae and neurological, psychological, endocrinologic, and other complications, although it remains unclear whether these symptoms originated from tumor location or treatment modality.
In conclusion, the patients with PA in our institute also showed a high survival rate similar to those in other institutions. The PFS of the patients with PA in our study only depended on the degree of surgical excision associated with the tumor location. As mentioned earlier, our study has some limitations. As it was retrospective and conducted in a single institute with a small number of patients, whether additional treatments for PA could increase the PFS rate is unclear. Despite these limitations, we think that this study is valuable because it is the first to investigate the long-term survival and prognosis of patients with PA in the Korean Yeungnam region. As shown in this study, although the survival rate was high in these patients, several medical sequelae and complications were experienced over the long term. Therefore, attention should be paid to managing the complications encountered by these long-term survivors. In addition, a Korean multicenter study is needed to evaluate the accurate prognosis of patients with PA.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download