1. Metellus P, Guyotat J, Chinot O, et al. Adult intracranial WHO grade II ependymomas: long-term outcome and prognostic factor analysis in a series of 114 patients. Neuro Oncol. 2010; 12:976–984. PMID:
20484442.

2. Mork SJ, Loken AC. Ependymoma: a follow-up study of 101 cases. Cancer. 1977; 40:907–915. PMID:
890671.
3. Guyotat J, Metellus P, Giorgi R, et al. Infratentorial ependymomas: prognostic factors and outcome analysis in a multi-center retrospective series of 106 adult patients. Acta Neurochir (Wien). 2009; 151:947–960. PMID:
19499166.

4. Jung TY, Jung S, Kook H, Baek HJ. Treatment decisions of World Health Organization grade II and III ependymomas in molecular era. J Korean Neurosurg Soc. 2018; 61:312–318. PMID:
29742878.

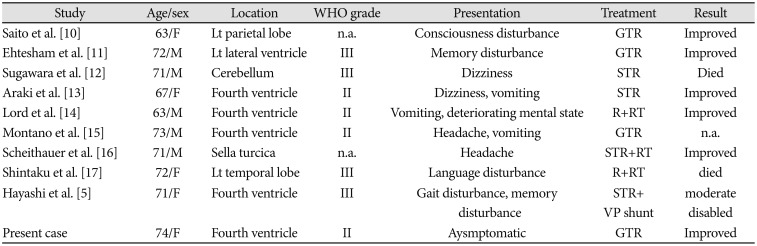
5. Hayashi T, Inamasu J, Kanai R, Sasaki H, Shinoda J, Hirose Y. Clinical, histological, and genetic features of fourth ventricle ependymoma in the elderly. Neurol Med Chir (Tokyo). 2012; 52:611–616. PMID:
22976148.

6. Ferguson SD, Levine NB, Suki D, et al. The surgical treatment of tumors of the fourth ventricle: a single-institution experience. J Neurosurg. 2018; 128:339–351. PMID:
28409732.

7. Amirian ES, Armstrong TS, Gilbert MR, Scheurer ME. Predictors of survival among older adults with ependymoma. J Neurooncol. 2012; 107:183–189. PMID:
21952907.

8. Jung HW, Yoo H, Paek SH, Choi KS. Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery. 2000; 46:567–574. discussion 574-5. PMID:
10719852.

9. Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol. 2006; 27:547–552. PMID:
16791048.

10. Saito T, Oki S, Mikami T, et al. [Supratentorial ectopic ependymoma: a case report]. No Shinkei Geka. 1999; 27:1139–1144. PMID:
10629896.
11. Ehtesham M, Kabos P, Yong WH, Schievink WI, Black KL, Yu JS. Development of an intracranial ependymoma at the site of a pre-existing cavernous malformation. Surg Neurol. 2003; 60:80–82. discussion 83. PMID:
12865022.

12. Sugawara T, Murakami R, Saito R, et al. [Two cases of ependymoma with atypical presentation]. Rinsho Hoshasen. 2003; 48:1218–1221.
13. Araki T, Shimono T, Kuwabara M, et al. [Two cases of ependymoma with atypical presentation]. Rinsho Hoshasen. 2008; 53:1141–1145.
14. Lord H, Ironside J, Summers D, Gregor A, Erridge S, Myles L. Fourth ventricle ependymoma in father and son. Br J Neurosurg. 2008; 22:423–425. PMID:
17952721.

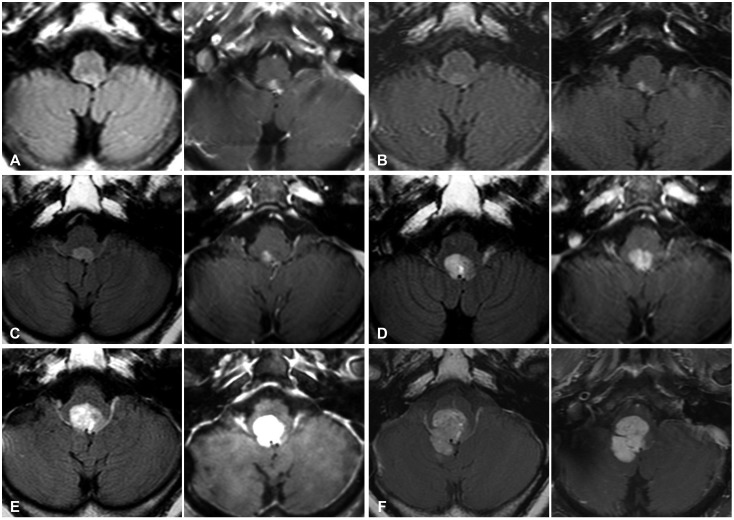
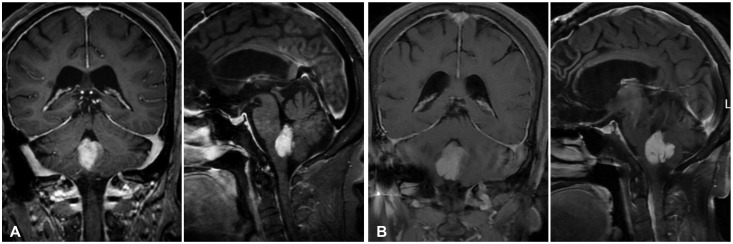
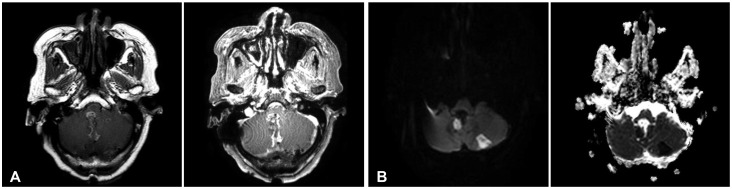
15. Montano N, De Bonis P, Doglietto F, et al. Teaching NeuroImage: hemorrhagic ependymoma in the elderly: a rare cause of headache and gait imbalance. Neurology. 2008; 70:e95. PMID:
18519865.

16. Scheithauer BW, Swearingen B, Whyte ET, Auluck PK, Stemmer-Rachamimov AO. Ependymoma of the sella turcica: a variant of pituicytoma. Hum Pathol. 2009; 40:435–440. PMID:
18992914.

17. Shintaku M, Hashimoto K. Anaplastic ependymoma simulating glioblastoma in the cerebrum of an adult. Brain Tumor Pathol. 2012; 29:31–36. PMID:
21833575.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download