Abstract
Background
Racial differences in American patients undergoing brain tumour surgery remain poorly characterized within urban medical centres. Our objective was to assess racial differences in operative brain tumour patients at a single academic hospital in Los Angeles, California.
Methods
We reviewed medical records of adult patients undergoing craniotomy for tumour resection from March 2013 to January 2017 at UCLA Medical Centre. Patients were categorized as Asian, Hispanic, Black, or White. Racial cohorts were matched on demographic variables for comparisons. Our primary outcome was post-operative length of stay (LOS). Secondary outcomes included hospital mortality and discharge disposition.
Results
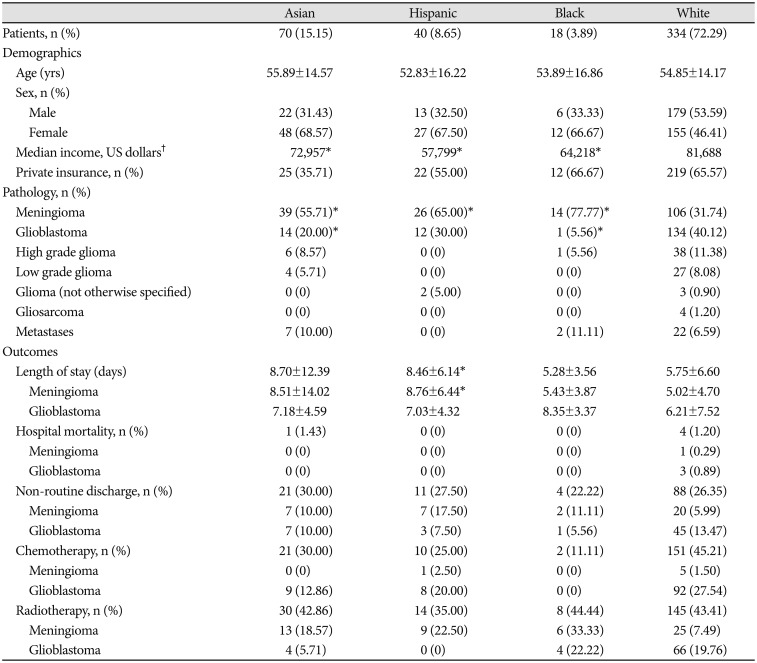
In this study, 462 patients identified as Asian (15.1%), Hispanic (8.7%), Black (3.9%), or White (72.3%). After cohort matching, non-White patients had elevated risk of prolonged LOS [odds ratio (OR)=2.62 (1.44, 4.76)]. No differences were observed in hospital mortality or non-routine discharge. Longer LOS was positively correlated with non-routine discharge [rpb (458)=0.41, p<0.001]. Black patients with government insurance had average LOS 2.84 days shorter than Black patients with private insurance (p=0.04). Among Hispanics, government insurance was associated with non-routine discharge [OR=4.93 (1.03, 24.00)].
Conclusion
Racial differences manifested as extended LOS for non-White patients, with comparable rates of hospital mortality and non-routine discharge across races. Prolonged LOS loosely reflected complicated clinical course with greater risk of adverse discharge disposition. Private insurance coverage predicted markedly lower risk of non-routine discharge for Hispanic patients, and LOS of three additional days among Black patients. Further research is needed to elucidate the basis of these differences.
Racial differences in health care outcomes among patients in the United States continue to be revealed in the literature [1234567891011,12]. It has been shown that minority patients have greater burdens of morbidity and mortality compared to White patients even after controlling for differences in socioeconomic status, education, and treatment [6781314151617181920212223]. Some studies attribute the origin of these differences to increased comorbidities in minority patients [2425262728] while others describe decreased access to care, disparate health care networks, distrust in the medical establishment, and doctor-centred biases as contributing to health care inequities [622293031].
Recent studies have focused on racial differences among Black patients diagnosed with brain tumours, but less work has been done with respect to other vulnerable populations. Asian and Hispanic people comprise a large subset of patients in many urban academic medical centres, yet have received less attention [67]. Moreover, previous research investigating racial differences in brain tumour patients has relied heavily on the use of national databases and multicentre survey studies [5782223]. Although helpful, the clinical granularity of such studies is limited. To our knowledge, the results of those studies have not been validated at the institutional level.
Here, we investigate outcomes of adult brain tumour patients who underwent craniotomy for tumour resection at a large academic medical centre in Los Angeles, California between March 2013 and January 2017. We chose hospital stay as our primary outcome measure. We also reviewed hospital mortality, discharge disposition, and delivery of adjuvant therapies at our institution (chemotherapy and radiotherapy). We assessed racial differences in these clinical measures across all patients, and again after identifying racial sub-cohorts matched on demographic and socioeconomic variables. We hypothesized that racial differences exist at the institutional level among adult patients undergoing brain tumour surgery, which may manifest as differences in length of stay (LOS) or rates of non-routine discharge.
We conducted a retrospective cross-sectional study that included adult patients (18 years or older) who underwent craniotomy for brain tumour resection at a single academic medical centre in Los Angeles, California from March 2013 to January 2017. Patients were selected using International Classification of Diseases, 9th and 10th Revision and Current Procedural Terminology, version 4 codes. Informed consent was obtained from all individual participants included in the study. The study had prior approval from our institutional review board and ethics committee before data collection was initiated (IRB approval number 17-000579).
Medical records were reviewed to identify race and to evaluate clinical variables of interest. Demographics (age, sex, race, Los Angeles County resident status, median income by zip code, and insurance status) and brain tumour pathologic diagnoses were also collected. We categorized patients into four major racial groups based on self-identification: Asian, Hispanic, Black, and White. Those patients whose race was reported as other, unknown, or those who refused to identify with a race were excluded. Median incomes were determined using the website https://www.incomebyzipcode.com. Insurance status was defined as government (including Medicaid, Medicare, or Tricare for U.S. military veterans) or nongovernment (i.e., private insurance or self-pay).
The primary outcome was hospital length of stay (LOS). Secondary outcome measures were hospital mortality, discharge disposition, and access to adjuvant therapies including chemotherapy and radiotherapy. LOS was also dichotomized and analysed as a categorical dependent variable where a prolonged LOS was defined as LOS greater than 7 days. Hospital mortality was defined as death occurring during hospitalization for brain tumour surgery. Discharge disposition was categorized as routine (to home) or non-routine (other than to home). Access to adjuvant therapies was determined through review of the medical records; only chemotherapy and/or radiotherapy for the brain tumour were considered.
We performed standard univariate analyses to compare outcomes of Asian, Hispanic, and Black patients to White patients. We performed additional comparisons stratified by specific brain tumour pathologies (meningioma and glioblastoma) within each of the four racial groups. Group-wise differences in continuous measures were identified using two-sample t tests. Variances were pooled between samples. However, if a violation of equal sample variances was detected based on Folded F tests, Satterthwaite's approximation for degrees of freedom was used to compute the p value. Statistical significance was determined a priori at an alpha level of 0.05.
Pearson (r) and point-biserial correlations (rpb) were performed to test for linear relationships between continuous/continuous and continuous/dichotomous variable pairs, respectively. We used the Mantel-Haenszel method [32] to test for bivariate associations between binary outcomes. Group-wise differences in binary measures were also analysed using odds ratio (OR), with 95% confidence interval (CI) for contingency tables evaluated using Fisher's exact tests. The above analyses were performed using SAS Studio (Cary, NC, USA).
We performed a secondary analysis with 1:1 matched cohorts, which matched Asian, Hispanic, and Black patients to White patients according to age, sex, Los Angeles County resident status, median income by zip code, and insurance status to isolate the effects of race on the outcomes of interest. This procedure was carried out through propensity score matching using the nearest neighbour approach [33] and was implemented using the “MatchIt” package in R (University of Auckland, Auckland, New Zealand).
A total of 517 adult patients were eligible. Of those, 55 patients who reported race as “other” or declined to identify with a race were excluded, leaving 462 patients who self-identified with a race and were included in the analysis. The study population included 70 patients who identified as Asian (15.1%), 40 Hispanic (8.7%), 18 Black (3.9%), and 334 White (72.3%). The racial breakdown of the overall cohort and the subset residing within Los Angeles County are depicted in Fig. 1, with comparison provided to the population demographics of Los Angeles reported in the 2010 U. S. Census.
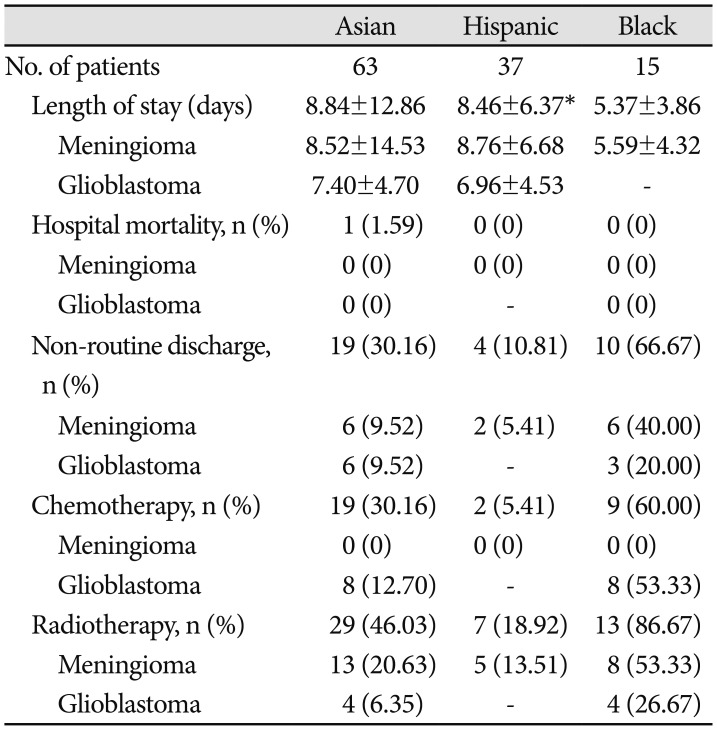
The mean age of patients was 54.8±14.5 years. Fifty-two percent of patients were female and 56% lived in Los Angeles County at the time of surgery. Meningioma was more frequently diagnosed in Asian, Hispanic, and Black patients (all p<0.001), whereas glioblastoma was less frequently diagnosed in Asian and Black patients when compared to White patients (all p=0.002). The results of our unmatched analyses are summarized in Table 1. The proportions of brain tumor pathology observed for patients of each race is also depicted graphically in Fig. 2. There were no significant differences in insurance status when we compared Asian, Hispanic, or Black patients to White patients. However, Asian (p=0.02), Hispanic (p<0.001), and Black (p=0.01) patients lived in residential areas associated with lower median incomes when compared to White patients, on average.
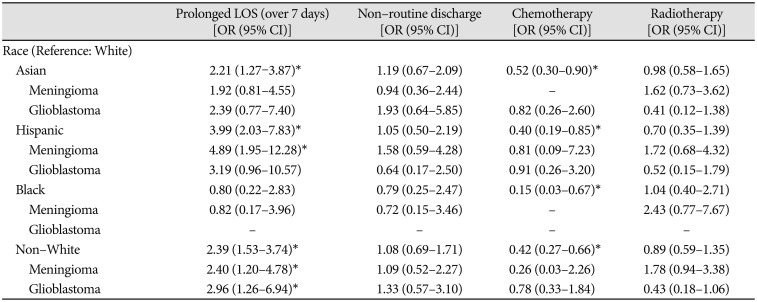
Across the entire cohort of 462 patients who identified as Asian, Hispanic, Black or White, the average LOS and 95% confidence interval was 6.41 (5.70, 7.11) days. Average LOS in patients stratified by race is tabulated in Table 1 and presented graphically in Fig. 3A. Average LOS was 8.70 (5.80, 11.61) days in Asians, 8.46 (6.56, 10.36) days for Hispanics, 5.28 (3.64, 6.93) days for Blacks, and 5.74 (5.04, 6.45) days for Whites. A standard Analysis of Variance (ANOVA) confirmed a significant main effect of patient race on LOS [F(3,458)=4.04, p=0.007]. Comparing specific racial groups, there was a higher average LOS in Asian compared to White patients, but the difference was not statistically significant (8.70 vs. 5.75 days, p=0.06). Asian patients had a significantly increased risk of prolonged LOS (beyond 7 hospital days). compared to White patients [OR 2.21, 95% CI (1.27–3.87)]. Hispanic patients stayed in the hospital on average longer than White patients (8.46 vs. 5.75 days, p<0.01).
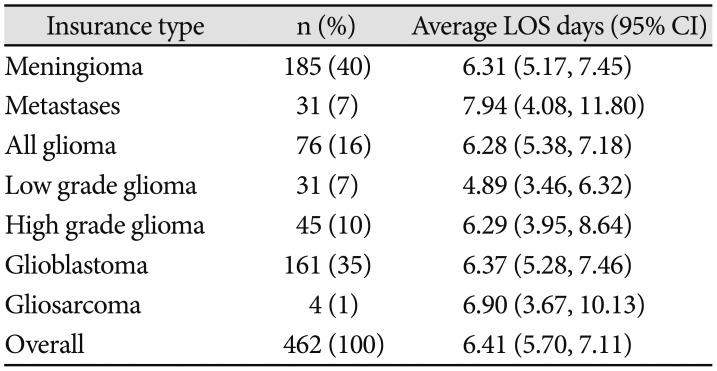
Table 2 and Fig. 3B compare LOS in patients stratified by brain tumor pathology. Overall, ANOVA demonstrated no main effect of brain tumor pathology on LOS [F(5,146)=0.496, p=0.78]. The longest LOS was observed following resection of intracranial metastases, with average LOS of 7.94 days (4.08, 11.80), while the shortest LOS of 4.89 days (3.46, 6.32) was observed following resection of low grade glioma. The most common brain, meningioma and glioblastoma, together consisted of 75% of all cases, and had similar LOS of 6.31 (5.17, 7.45) and 6.37 (5.28, 7.46) days, respectively.
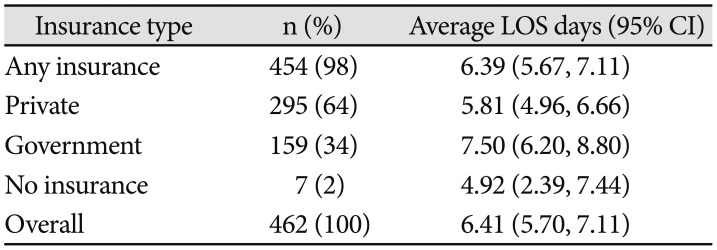
Comparison of LOS stratified by patients' medical insurance status is provided in Fig. 3C and Table 3. ANOVA revealed no significant effect of insurance type (private, government, or no insurance) on LOS, although a trend was observed [F(2,203)=2.56, p=0.078]. Average LOS for patients who had some form of billable health insurance for the inpatient stay was 6.39 (5.67, 7.11) days. Overall, patients with government insurance had the longest hospital stays on average [7.50 (6.20, 8.80) days], patients with private insurance had intermediate LOS [5.81 (4.96, 6.66) days], and patients with no registered health insurance had the shortest LOS [4.92 (2.39, 7.44) days].
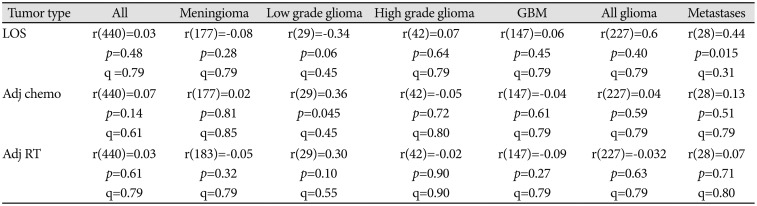
Next, we considered whether LOS was correlated with patient's age. Across all 462 patients in the cohort regardless of brain tumor pathology, we observed no significant correlation between age at surgery LOS [r(460)=0.05, p=0.28]. We also assessed for correlations between age and LOS stratified by each the specific type of tumor, but found no significant correlations between age and LOS for any of the tumor pathologies in our cohort (Table 4). Additionally, we assessed for possible correlations between LOS and patients' economic status, as approximated based upon the median income of patients' residential postal codes. There was no significant relationship between residential median income and LOS across patients [r(460)=0.05, p=0.28]. We further assessed correlations between residential median income and LOS within all tumor types with adequate sample size, and again found no significant correlations between LOS and residential median income (Table 5).
Longer average LOS in Hispanic patients relative to Whites was especially pronounced for patients diagnosed with meningiomas (8.76 vs. 5.02 days, p=0.001). For Hispanic patients diagnosed with a meningioma, higher median income was positively associated with prolonged LOS (rpb=0.42, p=0.03). Black patients with government insurance had post-operative hospital stays 2.84±1.86 days shorter on average than Black patients with private insurance coverage (p=0.04).
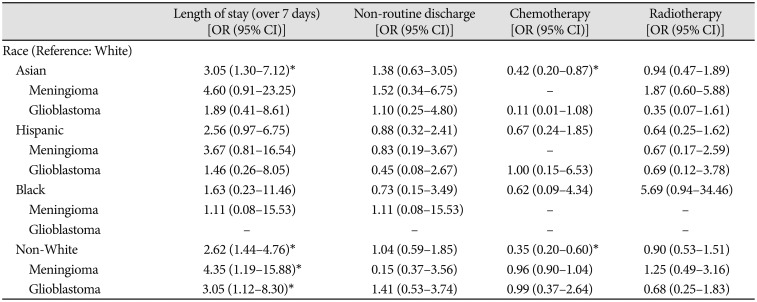
After cohort matching, Asian patients still had an increased risk of prolonged LOS [OR 3.05, 95% CI (1.30–7.12)], and Hispanic patients still stayed in the hospital 2.70±1.28 days longer than White patients (p=0.04). Collectively, non-White (Asian, Hispanic, and Black) patients had a higher risk of prolonged LOS whether pooling tumour types [OR 2.62, 95% CI (1.44–4.76)] or examining meningioma [OR 4.35, 95% CI (1.19–15.88)] or glioblastoma separately [OR 3.05, 95% CI (1.12–8.30)].
Across all patients, residential median income was not correlated with the probability of receiving adjuvant chemotherapy [r(440)=0.07, p=0.14] or adjuvant radiation therapy [r(440)=−0.02, p=0.61] within our health system, and no correlations between residential income and receipt of adjuvant therapy were observed when stratifying patients by tumor pathology (Table 5). There were no significant differences in hospital mortality or discharge disposition observed between races in either the unmatched (Table 5, 6) or matched (Table 7, 8) cohort analyses (see Methods for details of cohort matching). There were five cases of hospital mortality (1.1%) and 124 cases of non-routine discharge (26.8%). The most common types of non-routine discharge were to acute rehabilitation units (89 cases, 71.8%) or skilled nursing facilities (21 cases, 16.9%). For Hispanic patients, government insurance was associated with an increased risk of non-routine discharge [OR 4.93, 95% CI (1.03–24.00)]. For Black patients, higher median income was correlated with an increased risk of non-routine discharge (rpb=0.60, p=0.03).
For Hispanic patients diagnosed with glioblastoma, higher median income was positively associated with delivery of adjuvant radiotherapy at our institution (rpb=0.65, p=0.03). Among Hispanic patients diagnosed with glioblastoma, those treated with adjuvant radiotherapy at our institution had on average higher median incomes compared to patients not treated with adjuvant radiotherapy at our institution ($76,819 vs. $51,509, p=0.03). In the matched cohort analysis, Asian and non-White patients were less likely to receive chemotherapy at our institution [OR 0.42, 95% CI (0.20–0.87) and OR 0.35, 95% CI (0.20–0.60), respectively] compared to White patients. However, non-White patients were more likely to have diagnoses of benign tumors, while White patients had higher rates of high-grade gliomas and glioblastomas conventionally treated with adjuvant therapies (Fig. 1, Table 1).
Across patients of all patients, longer LOS was positively correlated with risk of a non-routine (i.e., non-home) discharge [rpb(458)=0.41, p<0.001]. Positive correlations between LOS and risk of non-routine discharge of similar magnitude were observed within Asian patients [rpb(68)=0.52, p<0.001], Black patients [rpb(16)=0.40, p=0.10], Hispanic patients [rpb(38)=0.56, p<0.001], White patients [rpb(330)=0.37, p<0.001], and all non-White patients [rpb(126)=0.41, p<0.001).
Recent work emphasizes delivery of equitable specialty care [53435]. With few exceptions, these studies have examined trends in morbidity and mortality in national databases such as the Nationwide Inpatient Sample [8], National Surgical Quality Improvement Program [7], and Surveillance, Epidemiology, and End Results registry [36]. Such analyses provide the advantage of large sample sizes generalizable to the American public. However, they are limited by possible coding errors and minimal clinical granularity, which renders them unable to elucidate differences in care within a specific city, health system, or institution.
Here, we identified racial differences in neurosurgical outcomes in adult patients who underwent craniotomy for brain tumour resection at a single academic hospital over four consecutive years. Both Asian patients and non-White patients in general had increased risks of prolonged LOS compared to White patients. Additionally, Hispanic patients had longer average LOS than White patients. Observed differences in LOS persisted in a matched cohort analysis controlling for demographic and socioeconomic variables, and were consistent with previous findings reported in the literature [822]. In the past decade, several national database studies of patients undergoing brain tumour resection have reported longer LOS in Black [7,82223] and Hispanic patients [7] compared to White patients. To our knowledge, however, ours is the first study to demonstrate such differences within patients treated at a single hospital.
Understanding the clinical significance of extended LOS is critical for interpreting racial differences in LOS among the operative patients in our study. One possibility is that patients with better insurance coverage or access to care are kept in hospital longer to recover adequately for home discharge, whereas patients with poorer coverage are discharged sooner with greater risk of adverse discharge disposition. In this scenario, longer LOS would be expected to correlate with higher rates of routine discharge. An alternative hypothesis is that prolonged LOS could reflect a poorer clinical course stemming from higher comorbidity burden or more advanced disease at presentation. This latter view is favoured by a majority of prior studies [6782223].
Under the hypothesis that extended LOS reflects heightened co-morbidity burden and disease severity, longer LOS would correlate with greater risk of non-routine discharge, such as discharge to a skilled nursing facility or acute rehabilitation unit. Conversely, patients with lower comorbidity burden or less advanced disease would have faster recovery timer (i.e., shorter LOS) and higher rates of home discharge. Indeed, previous outcome studies of operative brain tumour patients have reported the combination of longer LOS and higher rates of adverse discharge in minority patients [823]. Our own correlation analysis of LOS and discharge disposition was consistent with this framework: We observed weak yet consistently positive correlations (r=0.37–0.56) between LOS and non-routine discharge across patients of all racial demographics, with LOS explaining 17% of the overall variance in discharge disposition. This provides limited evidence that longer LOS in our patients was loosely correlated with adverse disposition (i.e., non-routine discharge), providing some context for which the observed racial differences in LOS might be viewed. While generally in line with the view of prior studies [6782223], this interpretation only serves as a rough generalization and falls short of addressing the full complexity of factors influencing LOS.
The lack of observed differences in LOS or non-routine discharge rates between Black and White patients in our study merits discussion. Prior studies of operative brain tumour patients, in contrast, have reported significant differences affecting Black patients, including higher overall morbidity [7242526], greater symptom severity at diagnosis [37], and extended LOS after surgery [678]. Unfortunately, our limited sample size of only 18 African-American patients severely limited our ability to draw definitive conclusions regarding outcomes in this regard. As LOS is a function of many clinical variables, it is difficult to interpret the potential meaning of our lack of observed differences. Nationwide data demonstrate greater severity of disease and increased comorbidity burden at surgery in Black patients. While these observations support higher expected rates of postoperative complications and longer LOS in Black patients [7], other factors could have simultaneously acted to shorten LOS. As yet one example, implicit racial biases affecting medical treatment of Black patients are described. These have been found to influence care decisions from pain management [3839] to administration of necessary procedures [40]. Though speculative, it is conceivable that such implicit biases could also affect clinicians' discharge decisions in a postoperative context. Whether such biases affect LOS in patients undergoing brain tumour resection remains unknown and requires further investigation.
Other factors that may have influenced the observed outcome measures in a race-dependent manner relate to racial differences in patients' decisions to seek or accept treatment. Such decisions could be related to difficulty accessing care, disparate health care networks and quality of care, distrust in the medical establishment, or doctor-centred biases [622293031]. Additionally, differences in cultural values or beliefs may have affected patients' decisions regarding their care, and could have affected the duration for which patients wished to remain in hospital postoperatively [4142]. It is unclear to what extent such factors influenced outcomes in our study, as we did not investigate underlying circumstances of discharge (e.g., to what extent patients dictated discharge times or whether patients left against medical advice). The decision to leave hospital care could also reflect socioeconomic differences given the increased costs of extended LOS to the patient. This hypothesis seems plausible given that Black patients in our study who had access to private insurance remained in the hospital for nearly three more post-operative days than Black patients who had government insurance.
The racial differences highlighted in our study should be addressed through a combination of patient education, clinician awareness of implicit bias, and preventative health care initiatives that prioritize equitable access to quality health care. To this end, more research is needed to ascertain the extent of racial differences in baseline morbidity among our cohort. Identifying the specific comorbidities most associated with outcome differences among our patient population could help prioritize public health efforts within our institution's health system and through collaborative efforts with outside providers. Further investigation should also consider how implicit bias among medical decision-makers may relate to LOS or treatment decisions. Finally, patient surveys could shed light on racial, cultural, or socioeconomic differences that affect patients' trust in their healthcare providers or their goals of care.
Several limitations affect the conclusions of our study. First, our study is retrospective and limited by demographic information available in the medical record. Several other patient characteristics may have impacted outcomes but were not retrievable in retrospect. In particular, patient income data was based on the median income of patients' residential zip codes, which is less accurate than patient-reported income. Median incomes by zip code for Black and Hispanic patients were $64,218 and $57,799 on average. Both numbers surpass the median household income of $55,909 for Los Angeles County. These numbers are likely higher than incomes for the majority of Black and Hispanic patients receiving care in Los Angeles County, and our surrogate income measures may have over-estimated the true incomes of the Black and Hispanic patients included in our study.
Second, our sample size was significantly limited for patients of non-White racial backgrounds, especially for Black (n=18) and Hispanic (n=40) patients. This reduced our ability to sub-stratify racial cohorts by clinical or demographic variables, and limited our statistical power. Third, we did not characterize comorbid status or disease severity at the time of surgery, both of which could have profound effects on patient outcome and hospital LOS. Fourth, our data on rates of adjuvant therapy was limited to adjuvant treatments administered at our institution. In many cases, adjuvant therapy is provided at outside institutions closer to the patient's home, and the likelihood of receiving adjuvant therapy at our institution versus outside centres may have differed between races. Thus, our data on adjuvant therapies must be interpreted with caution.
Finally, it is important to note that our single-institution study does not address the full range of racial differences observed for patients in our vicinity undergoing brain tumour surgery (i.e., Los Angeles County) [678]. Only 3.9% of the patients in our cohort were Black, despite Black persons comprising 9.8% of Los Angeles County. Similarly, 8.7% of our cohort identified as Hispanic even though Hispanics currently comprise 47% of the population of Los Angeles County. A comprehensive assessment of racial differences affecting brain tumour patients in our area would require detailed institutional data from other nearby hospitals serving Los Angeles county. Despite these limitations, our study is the first to describe racial differences in adult brain tumour patients treated at a single institution and adds to the growing literature [57822233135].
In conclusion, we conducted a single-institution study on racial differences for adult patients undergoing surgical resection of brain tumours. There were no racial differences in rates of hospital mortality or non-routine discharge. Thus, we found no evidence of racial differences in short-term survival or functional outcomes. However, we found a significant main effect of race on hospital LOS, and higher incidence of prolonged LOS in non-White relative to White patients. Asian and Hispanic patients stayed in the hospital longer than White and Black patients following surgery. Differences in LOS remained significant after matching cohorts on demographic and socioeconomic variables. Although there were no differences in non-routine discharge, we did observe a significant positive correlation between longer LOS and non-routine discharge across our cohort.
In contrast to race, other factors we considered likely to affect outcomes, including brain tumor pathology, age at surgery, insurance status, and estimated income, were not significantly associated with length of stay across patients. However, access to private insurance notably affected outcomes for non-White patients. Hispanic patients lacking private insurance had nearly 5 times the odds of non-routine discharge as Hispanic patients with private insurance coverage, and Black patients with government insurance remained in hospital for nearly 3 fewer post-operative days than Black patients with private insurance coverage. Moreover, Black and Hispanic patients were markedly underrepresented in our cohort relative to the demographic composition of Los Angeles County.
In Los Angeles as in the United States as a whole, healthcare outcomes and access to quality care are disparate along socioeconomic and racial lines. While racial background is a nonmodifiable factor, investigating racial differences in surgical outcomes and understanding their root causes is, in our opinion, critical. In terms of its effect on length of hospital stay, race outweighed all other factors considered, including brain tumor pathology, age at surgery, insurance status, and estimated income. Racial differences in neurosurgical patients and their clinical outcomes should continue to be evaluated at the institutional level to better understand current challenges and barriers to care. Longer-term goals of this research are to further characterize racial disparities among neurosurgical patients, assess whether and how institutional or other systems-level factors may contribute to such differences, and to provide insights into systems-level changes that might be implemented to address disparities and achieve the best possible neurosurgical outcomes for all our patients regardless of race.
Acknowledgments
Methma Udawatta, John P. Sheppard and Thien Nguyen are recipients of the David Geffen Medical Scholarship. Carlito Lagman and Prasanth Romiyo have received support from the Tina and Fred Segal Benign Brain Tumor and Skull Base Research Fellowship. Isaac Yang is supported by the UCLA Visionary Ball Fund Grant, Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research UCLA Scholars in Translational Medicine Program Award, Jason Dessel Memorial Seed Grant, UCLA Honberger Endowment Brain Tumor Research Seed Grant, and Stop Cancer (US) Research Career Development Award.
References
1. Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002; 94:666–668. PMID: 12152921.
2. Institute of Medicine (US) Committee on Cancer Research Among Minorities and the Medically Underserved. Haynes MA, Smedley BD. The unequal burden of cancer: an assessment of NIH research and programs for ethnic minorities and the medically underserved. Washington, DC: National Academies Press;1999.
3. Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002; 94:334–357. PMID: 11880473.

4. Geronimus AT, Bound J, Waidmann TA, Hillemeier MM, Burns PB. Excess mortality among blacks and whites in the United States. N Engl J Med. 1996; 335:1552–1558. PMID: 8900087.

5. Curry WT Jr, Barker FG 2nd. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009; 93:25–39. PMID: 19430880.

6. Curry WT Jr, Carter BS, Barker FG 2nd. Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988-2004. Neurosurgery. 2010; 66:427–437. PMID: 20124933.

7. Dasenbrock HH, Liu KX, Devine CA, et al. Length of hospital stay after craniotomy for tumor: a National Surgical Quality Improvement Program analysis. Neurosurg Focus. 2015; 39:E12.

8. Muhlestein WE, Akagi DS, Chotai S, Chambless LB. The impact of race on discharge disposition and length of hospitalization after craniotomy for brain tumor. World Neurosurg. 2017; 104:24–38. PMID: 28478245.

9. Zhang W, Lyman S, Boutin-Foster C, et al. Racial and ethnic disparities in utilization rate, hospital volume, and perioperative outcomes after total knee arthroplasty. J Bone Joint Surg Am. 2016; 98:1243–1252. PMID: 27489314.

10. Damle RN, Flahive JM, Davids JS, Maykel JA, Sturrock PR, Alavi K. Examination of racial disparities in the receipt of minimally invasive surgery among a national cohort of adult patients undergoing colorectal surgery. Dis Colon Rectum. 2016; 59:1055–1062. PMID: 27749481.

11. Taioli E, Wolf AS, Camacho-Rivera M, et al. Racial disparities in esophageal cancer survival after surgery. J Surg Oncol. 2016; 113:659–664. PMID: 26865174.

12. Lucas FL, Stukel TA, Morris AM, Siewers AE, Birkmeyer JD. Race and surgical mortality in the United States. Ann Surg. 2006; 243:281–286. PMID: 16432363.

13. Ragland KE, Selvin S, Merrill DW. Black-white differences in stage-specific cancer survival: analysis of seven selected sites. Am J Epidemiol. 1991; 133:672–682. PMID: 2018022.
14. Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994; 272:947–954. PMID: 8084062.

15. Yood MU, Johnson CC, Blount A, et al. Race and differences in breast cancer survival in a managed care population. J Natl Cancer Inst. 1999; 91:1487–1491. PMID: 10469750.

16. Optenberg SA, Thompson IM, Friedrichs P, Wojcik B, Stein CR, Kramer B. Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA. 1995; 274:1599–1605. PMID: 7474244.

17. Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer. 1999; 85:485–491. PMID: 10023719.
18. Surawicz TS, Davis F, Freels S, Laws ER Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998; 40:151–160. PMID: 9892097.
19. Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973-1991. J Neurosurg. 1998; 88:1–10. PMID: 9420066.

20. Polednak AP, Flannery JT. Brain, other central nervous system, and eye cancer. Cancer. 1995; 75(1 Suppl):330–337. PMID: 8001004.

21. Shin JY, Yoon JK, Diaz AZ. Racial disparities in anaplastic oligodendroglioma: an analysis on 1643 patients. J Clin Neurosci. 2017; 37:34–39. PMID: 28024733.

22. Mukherjee D, Patil CG, Todnem N, et al. Racial disparities in Medicaid patients after brain tumor surgery. J Clin Neurosci. 2013; 20:57–61. PMID: 23084348.

23. Nuño M, Mukherjee D, Elramsisy A, et al. Racial and gender disparities and the role of primary tumor type on inpatient outcomes following craniotomy for brain metastases. Ann Surg Oncol. 2012; 19:2657–2663. PMID: 22618715.

24. Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002; 287:2106–2113. PMID: 11966385.

25. Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst. 2001; 93:501–515. PMID: 11287444.

26. West DW, Satariano WA, Ragland DR, Hiatt RA. Comorbidity and breast cancer survival: a comparison between black and white women. Ann Epidemiol. 1996; 6:413–419. PMID: 8915472.

27. Sutherland CM, Mather FJ. Long-term survival and prognostic factors in breast cancer patients with localized (no skin, muscle, or chest wall attachment) disease with and without positive lymph nodes. Cancer. 1986; 57:622–629. PMID: 3942999.

28. Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994; 120:104–110. PMID: 8256968.

29. Masi CM, Gehlert S. Perceptions of breast cancer treatment among African-American women and men: implications for interventions. J Gen Intern Med. 2009; 24:408–414. PMID: 19101776.

30. Haider AH, Sexton J, Sriram N, et al. Association of unconscious race and social class bias with vignette-based clinical assessments by medical students. JAMA. 2011; 306:942–951. PMID: 21900134.

31. Nooka AK, Behera M, Lonial S, Dixon MD, Ramalingam SS, Pentz RD. Access to Children's Oncology Group and Pediatric Brain Tumor Consortium phase 1 clinical trials: Racial/ethnic dissimilarities in participation. Cancer. 2016; 122:3207–3214. PMID: 27404488.

32. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959; 22:719–748. PMID: 13655060.
33. Ho D, Imai K, King G, Stuart E. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007; 15:199–326.

34. Mukherjee D, Zaidi HA, Kosztowski T, et al. Disparities in access to neuro-oncologic care in the United States. Arch Surg. 2010; 145:247–253. PMID: 20231625.

35. Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer. 2003; 98:603–609. PMID: 12879479.

36. Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973-2001. Cancer. 2006; 106:1358–1363. PMID: 16470608.
37. Byers TE, Wolf HJ, Bauer KR, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008; 113:582–591. PMID: 18613122.
38. Singhal A, Tien YY, Hsia RY. Racial-ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS One. 2016; 11:e0159224. PMID: 27501459.

39. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016; 113:4296–4301. PMID: 27044069.

40. Harris DR, Andrews R, Elixhauser A. Racial and gender differences in use of procedures for black and white hospitalized adults. Ethn Dis. 1997; 7:91–105. PMID: 9386949.
41. Chaturvedi SK, Strohschein FJ, Saraf G, Loiselle CG. Communication in cancer care: psycho-social, interactional, and cultural issues. A general overview and the example of India. Front Psychol. 2014; 5:1332. PMID: 25452741.

Fig. 1
Racial composition of patients undergoing craniotomy for tumor resection. A: All craniotomy patients within our institutional study. B: Craniotomy patients within our study residing in Los Angeles County at time of surgery. C: Racial demographics of Los Angeles County reported in 2010 United States Census.
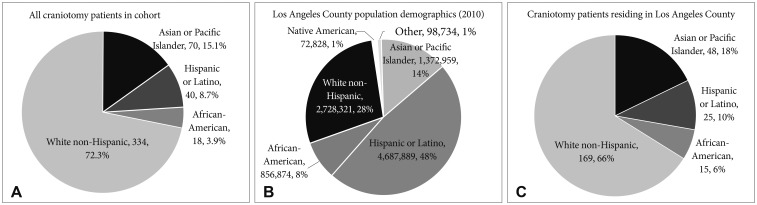
Fig. 2
Relative frequencies of brain tumor pathologies by race in patients undergoing craniotomy for tumor resection at our institution.
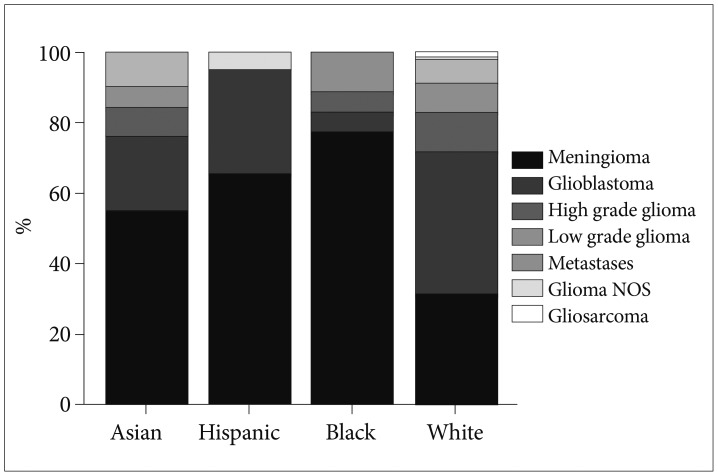
Fig. 3
Comparisons of average hospital length of stay in patients undergoing craniotomy for tumor resection stratified by (A) patient race, (B) brain tumor pathology, and (C) type of medical insurance. Error bars indicate 95% confidence intervals.
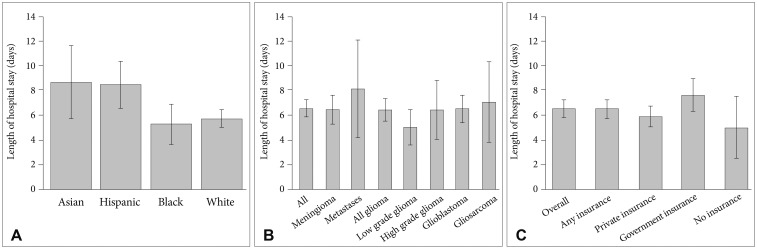
Table 5
Correlations between median income and length of stay, probability of receiving adjuvant chemotherapy, and probability of receiving adjuvant radiation therapy within our health system following surgical resection, stratified by type of brain tumor




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download