1. Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006; 176:2414–2422. discussion 2422. PMID:
17085118.

2. Challacombe BJ, Bochner BH, Dasgupta P, Gill I, Guru K, Herr H, et al. The role of laparoscopic and robotic cystectomy in the management of muscle-invasive bladder cancer with special emphasis on cancer control and complications. Eur Urol. 2011; 60:767–775. PMID:
21620562.

3. Guiote I, Gaya JM, Gausa L, Rodríguez O, Palou J. Complications from robot-assisted radical cystectomy: where do we stand. Actas Urol Esp. 2016; 40:108–114. PMID:
25914065.

4. Parekh DJ, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018; 391:2525–2536. PMID:
29976469.
5. Bochner BH, Dalbagni G, Sjoberg DD, Silberstein J, Keren Paz GE, Donat SM, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol. 2015; 67:1042–1050. PMID:
25496767.

6. Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, El-Tabey NA, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int. 2003; 92:232–236. PMID:
12887473.

7. Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010; 57:196–201. PMID:
19853987.

8. Bachman AG, Parker AA, Shaw MD, Cross BW, Stratton KL, Cookson MS, et al. Minimally invasive versus open approach for cystectomy: trends in the utilization and demographic or clinical predictors using the National Cancer Database. Urology. 2017; 103:99–105. PMID:
28214574.

9. Hanna N, Leow JJ, Sun M, Friedlander DF, Seisen T, Abdollah F, et al. Comparative effectiveness of robot-assisted vs. open radical cystectomy. Urol Oncol. 2018; 36:88.e1–88.e9.

10. Parekh DJ, Messer J, Fitzgerald J, Ercole B, Svatek R. Perioperative outcomes and oncologic efficacy from a pilot prospective randomized clinical trial of open versus robotic assisted radical cystectomy. J Urol. 2013; 189:474–479. PMID:
23017529.

11. Khan MS, Gan C, Ahmed K, Ismail AF, Watkins J, Summers JA, et al. A single-centre early phase randomised controlled three-arm trial of open, robotic, and laparoscopic radical cystectomy (CORAL). Eur Urol. 2016; 69:613–621. PMID:
26272237.

12. Parekh D. Pnflba-18: a prospective, multicenter, randomized trial of open versus robotic radical cystectomy (RAZOR). J Urol. 2017; 197:e918.

13. Augestad KM, Delaney CP. Postoperative ileus: impact of pharmacological treatment, laparoscopic surgery and enhanced recovery pathways. World J Gastroenterol. 2010; 16:2067–2074. PMID:
20440846.

14. Kang SG, Kang SH, Lee YG, Rha KH, Jeong BC, Ko YH, et al. Robot-assisted radical cystectomy and pelvic lymph node dissection: a multi-institutional study from Korea. J Endourol. 2010; 24:1435–1440. PMID:
20839973.

15. Kang SG, Ko YH, Jang HA, Kim J, Kim SH, Cheon J, et al. Initial experience of robot-assisted radical cystectomy with total intracorporeal urinary diversion: comparison with extracorporeal method. J Laparoendosc Adv Surg Tech A. 2012; 22:456–462. PMID:
22462649.

16. Ali-El-Dein B, Gomha M, Ghoneim MA. Critical evaluation of the problem of chronic urinary retention after orthotopic bladder substitution in women. J Urol. 2002; 168:587–592. PMID:
12131315.

17. Niver BE, Daneshmand S, Satkunasivam R. Female reproductive organ-sparing radical cystectomy: contemporary indications, techniques and outcomes. Curr Opin Urol. 2015; 25:105–110. PMID:
25581538.
18. Herr H, Lee C, Chang S, Lerner S. Bladder Cancer Collaborative Group. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: a collaborative group report. J Urol. 2004; 171:1823–1828. discussion 1827-8. PMID:
15076285.

19. Wilson TG, Guru K, Rosen RC, Wiklund P, Annerstedt M, Bochner BH, et al. Pasadena Consensus Panel. Best practices in robot-assisted radical cystectomy and urinary reconstruction: recommendations of the Pasadena Consensus Panel. Eur Urol. 2015; 67:363–375. PMID:
25582930.

20. Yuh B, Wilson T, Bochner B, Chan K, Palou J, Stenzl A, et al. Systematic review and cumulative analysis of oncologic and functional outcomes after robot-assisted radical cystectomy. Eur Urol. 2015; 67:402–422. PMID:
25560797.

21. Hautmann RE, de Petriconi RC, Volkmer BG. 25 years of experience with 1,000 neobladders: long-term complications. J Urol. 2011; 185:2207–2212. PMID:
21497841.

22. Lee RK, Abol-Enein H, Artibani W, Bochner B, Dalbagni G, Daneshmand S, et al. Urinary diversion after radical cystectomy for bladder cancer: options, patient selection, and outcomes. BJU Int. 2014; 113:11–23. PMID:
24330062.

23. Hautmann RE, Abol-Enein H, Davidsson T, Gudjonsson S, Hautmann SH, Holm HV, et al. International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer 2012. ICUD-EAU International Consultation on Bladder Cancer 2012: urinary diversion. Eur Urol. 2013; 63:67–80. PMID:
22995974.
24. Novara G, Ficarra V, Minja A, De Marco V, Artibani W. Functional results following vescica ileale Padovana (VIP) neobladder: midterm follow-up analysis with validated questionnaires. Eur Urol. 2010; 57:1045–1051. PMID:
20096993.

25. Koie T, Hatakeyama S, Yoneyama T, Ishimura H, Yamato T, Ohyama C. Experience and functional outcome of modified ileal neobladder in 95 patients. Int J Urol. 2006; 13:1175–1179. PMID:
16984548.

26. Nayak AL, Cagiannos I, Lavallée LT, Morash C, Hickling D, Mallick R, et al. Urinary function following radical cystectomy and orthotopic neobladder urinary reconstruction. Can Urol Assoc J. 2018; 12:181–186. PMID:
29485037.

27. Schwentner C, Sim A, Balbay MD, Todenhöfer T, Aufderklamm S, Halalsheh O, et al. Robot-assisted radical cystectomy and intracorporeal neobladder formation: on the way to a standardized procedure. World J Surg Oncol. 2015; 13:3. PMID:
25560783.

28. Simone G, Papalia R, Misuraca L, Tuderti G, Minisola F, Ferriero M, et al. Robotic intracorporeal padua ileal bladder: surgical technique, perioperative, oncologic and functional outcomes. Eur Urol. 2018; 73:934–940. PMID:
27780643.

29. Jonsson MN, Adding LC, Hosseini A, Schumacher MC, Volz D, Nilsson A, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion in patients with transitional cell carcinoma of the bladder. Eur Urol. 2011; 60:1066–1073. PMID:
21852033.

30. Tyritzis SI, Hosseini A, Collins J, Nyberg T, Jonsson MN, Laurin O, et al. Oncologic, functional, and complications outcomes of robot-assisted radical cystectomy with totally intracorporeal neobladder diversion. Eur Urol. 2013; 64:734–741. PMID:
23768634.

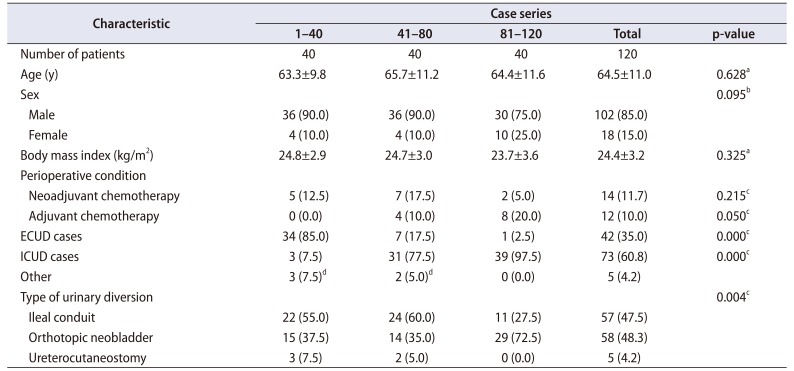
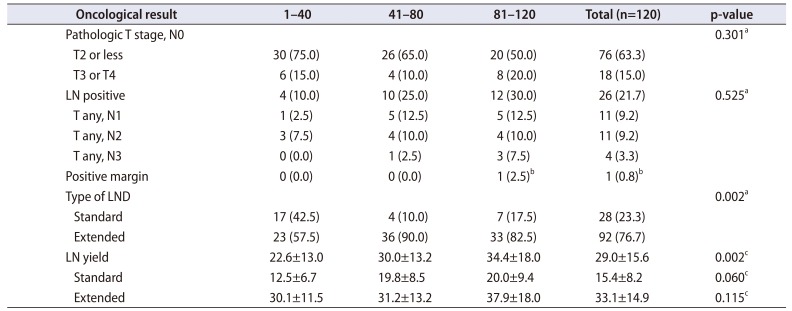
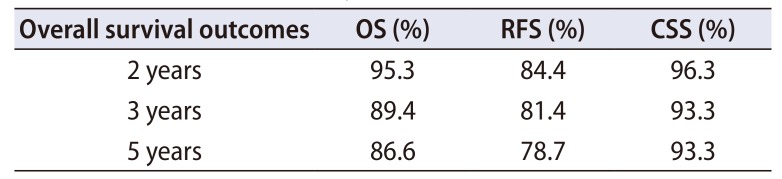
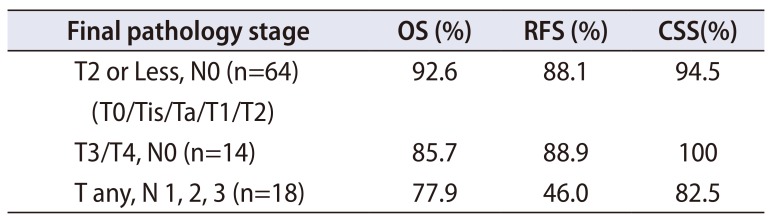




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download