Abstract
Mesenchymal stem cells (MSCs) ameliorate the renal injury in Adriamycin (ADR)-induced nephropathy, but the mechanisms underlying their efficacy remain incompletely understood. In this study, we demonstrated that MSCs increased the survival, recovered body weight loss, and decreased proteinuria and serum creatinine levels in ADR-treated mice. MSCs also prevented podocyte damage and renal fibrosis by decreasing the expression of fibronectin, collagen 1α1, and α-smooth muscle actin. From a mechanistic perspective, MSCs inhibited renal inflammation by lowering the expression of CCL4, CCL7, CCL19, IFN-α/β, TGF-β, TNF-α, and chitinase 3-like 1. In summary, our data demonstrate that MSCs improve renal functions by inhibiting renal inflammation in ADR-induced nephropathy.
References
1. Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton). 2011; 16:30–38.

2. Nogueira A, Pires MJ, Oliveira PA. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In Vivo. 2017; 31:1–22.

3. Zhang H, Ren R, Du J, Sun T, Wang P, Kang P. AF1q contributes to Adriamycin-induced podocyte injury by activating Wnt/β-catenin signaling. Kidney Blood Press Res. 2017; 42:794–803.

4. Zoja C, Garcia PB, Rota C, Conti S, Gagliardini E, Corna D, Zanchi C, Bigini P, Benigni A, Remuzzi G, et al. Mesenchymal stem cell therapy promotes renal repair by limiting glomerular podocyte and progenitor cell dysfunction in Adriamycin-induced nephropathy. Am J Physiol Renal Physiol. 2012; 303:F1370–F1381.

5. Huuskes BM, Wise AF, Cox AJ, Lim EX, Payne NL, Kelly DJ, Samuel CS, Ricardo SD. Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. FASEB J. 2015; 29:540–553.

6. Mahajan D, Wang Y, Qin X, Wang Y, Zheng G, Wang YM, Alexander SI, Harris DC. CD4+ CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol. 2006; 17:2731–2741.

7. Najar M, Krayem M, Meuleman N, Bron D, Lagneaux L. Mesenchymal stromal cells and Toll-like receptor priming: a critical review. Immune Netw. 2017; 17:89–102.

8. Ma H, Wu Y, Zhang W, Dai Y, Li F, Xu Y, Wang Y, Tu H, Li W, Zhang X. The effect of mesenchymal stromal cells on doxorubicin-induced nephropathy in rats. Cytotherapy. 2013; 15:703–711.

9. Fitzsimmons RE, Mazurek MS, Soos A, Simmons CA. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018; 2018:8031718.

10. Ma H, Wu Y, Xu Y, Sun L, Zhang X. Human umbilical mesenchymal stem cells attenuate the progression of focal segmental glomerulosclerosis. Am J Med Sci. 2013; 346:486–493.

11. Yokote S, Katsuoka Y, Yamada A, Ohkido I, Yokoo T. Effect of adipose-derived mesenchymal stem cell transplantation on vascular calcification in rats with adenine-induced kidney disease. Sci Rep. 2017; 7:14036.

12. Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005; 68:1613–1617.

13. Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004; 15:1794–1804.

14. Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007; 18:2486–2496.

15. Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007; 292:F1626–F1635.

16. Lee HK, Kim HS, Kim JS, Kim YG, Park KH, Lee JH, Kim KH, Chang IY, Bae SC, Kim Y, et al. CCL2 deficient mesenchymal stem cells fail to establish long-lasting contact with T cells and no longer ameliorate lupus symptoms. Sci Rep. 2017; 7:41258.

17. Kim DH, Park HJ, Lim S, Koo JH, Lee HG, Choi JO, Oh JH, Ha SJ, Kang MJ, Lee CM, et al. Regulation of chitinase-3-like-1 in T cell elicits Th1 and cytotoxic responses to inhibit lung metastasis. Nat Commun. 2018; 9:503.

18. Wang Y, Wang YP, Tay YC, Harris DC. Progressive Adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000; 58:1797–1804.

19. Lee YH, Seo JW, Kim YG, Moon JY, Kim JS, Jeong KH, Kim BM, Kim KW, Yang CW, Kim CD, et al. Validation study of an operational tolerance signature in Korean kidney transplant recipients. Immune Netw. 2018; 18:e36.

20. Peng X, Xu H, Zhou Y, Wang B, Yan Y, Zhang X, Wang M, Gao S, Zhu W, Xu W, et al. Human umbilical cord mesenchymal stem cells attenuate cisplatin-induced acute and chronic renal injury. Exp Biol Med (Maywood). 2013; 238:960–970.

21. Uehara Y, Numabe A, Hirawa N, Kawabata Y, Iwai J, Ono H, Matsuoka H, Takabatake Y, Yagi S, Sugimoto T. Antihypertensive effects of cicletanine and renal protection in Dahl salt-sensitive rats. J Hypertens. 1991; 9:719–728.

22. Murakami A, Oshiro H, Kanzaki S, Yamaguchi A, Yamanaka S, Furuya M, Miura S, Kanno H, Nagashima Y, Aoki I, et al. A novel method for isolating podocytes using magnetic activated cell sorting. Nephrol Dial Transplant. 2010; 25:3884–3890.

23. Lee HK, Kim KH, Kim HS, Kim JS, Lee JH, Ji A, Kim KS, Lee TY, Chang IY, Bae SC, et al. Effect of a combination of prednisone or mycophenolate mofetil and mesenchymal stem cells on lupus symptoms in MRL. Fas lpr mice. Stem Cells Int. 2018; 2018:4273107.
24. Lee JH, Lee HK, Kim HS, Kim JS, Ji AY, Lee JS, Kim KS, Lee TY, Bae SC, Kim Y, et al. CXCR3-deficient mesenchymal stem cells fail to infiltrate into the nephritic kidney and do not ameliorate lupus symptoms in MRL. Fas lpr mice. Lupus. 2018; 27:1854–1859.

25. Montgomery TA, Xu L, Mason S, Chinnadurai A, Lee CG, Elias JA, Cantley LG. Breast regression protein-39/chitinase 3-like 1 promotes renal fibrosis after kidney injury via activation of myofibroblasts. J Am Soc Nephrol. 2017; 28:3218–3226.

26. Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009; 27:3063–3073.

27. Wise AF, Ricardo SD. Mesenchymal stem cells in kidney inflammation and repair. Nephrology (Carlton). 2012; 17:1–10.

28. Wu HJ, Yiu WH, Li RX, Wong DW, Leung JC, Chan LY, Zhang Y, Lian Q, Lin M, Tse HF, et al. Mesenchymal stem cells modulate albumin-induced renal tubular inflammation and fibrosis. PLoS One. 2014; 9:e90883.

29. Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011; 73:479–501.

30. Nielsen TL, Plesner LL, Warming PE, Pallisgaard JL, Dalsgaard M, Schou M, Høst U, Rydahl C, Brandi L, Køber L, et al. YKL-40 in patients with end-stage renal disease receiving haemodialysis. Biomarkers. 2018; 23:357–363.

31. Nadkarni GN, Rao V, Ismail-Beigi F, Fonseca VA, Shah SV, Simonson MS, Cantley L, Devarajan P, Parikh CR, Coca SG. Association of urinary biomarkers of inflammation, injury, and fibrosis with renal function decline: the ACCORD trial. Clin J Am Soc Nephrol. 2016; 11:1343–1352.

32. Ziatabar S, Zepf J, Rich S, Danielson BT, Bollyky PI, Stern R. Chitin, chitinases, and chitin lectins: emerging roles in human pathophysiology. Pathophysiology. 2018; 25:253–262.

33. Wang S, Li Y, Zhao J, Zhang J, Huang Y. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant. 2013; 19:538–546.

34. Zhong F, Wang W, Lee K, He JC, Chen N. Role of C/EBP-α in Adriamycin-induced podocyte injury. Sci Rep. 2016; 6:33520.

35. Szalay CI, Erdélyi K, Kökény G, Lajtár E, Godó M, Révész C, Kaucsár T, Kiss N, Sárközy M, Csont T, et al. Oxidative/nitrative stress and inflammation drive progression of doxorubicin-induced renal fibrosis in rats as revealed by comparing a normal and a fibrosis-resistant rat strain. PLoS One. 2015; 10:e0127090.

Figure 1.
MSCs ameliorate renal injury in ADR-treated mice. (A-G) Male BALB/c mice were intravenously injected with 10 mg/kg of ADR on day 0, and MSCs (1×106 cells/mouse) on days 7 and 14 (arrows). Mice were sacrificed on day 28 to determine (A) survival rate, (B) body weight, (C) kidney morphology and weight, (D) proteinuria level, and (E) serum creatinine level. (F) Kidney sections were stained with PAS or Masson's trichrome stains. The amounts of glycoprotein (arrow ①), the size of capsular spaces (arrow ②), and the size of tubules (arrow ③), and the amounts of collagens (arrow ④) in the glomeruli were examined. Glomerular size, glomerular sclerotic index, and tubular damage score are presented. (G) Expression of the podocyte injury marker desmin was measured by immunohistochemistry. Each group are presented as CON (chemically-untreated control mice; n=6), ADR (ADR-treated mice; n=6), and ADR+MSC (ADR and MSC-treated mice; n=7), respectively. (H) Podocytes were isolated from normal BALB/c mice and were cultured with MSCs and/or 0.5 µg/ml of ADR for 24 h. Gene and protein expression of apoptotic (BAX) and anti-apoptotic (Bcl-2) molecules were measured by RT-qPCR and Western blot, respectively. * p<0.05 CON versus ADR; † p<0.05 ADR versus ADR+MSC; ‡ p<0.05.
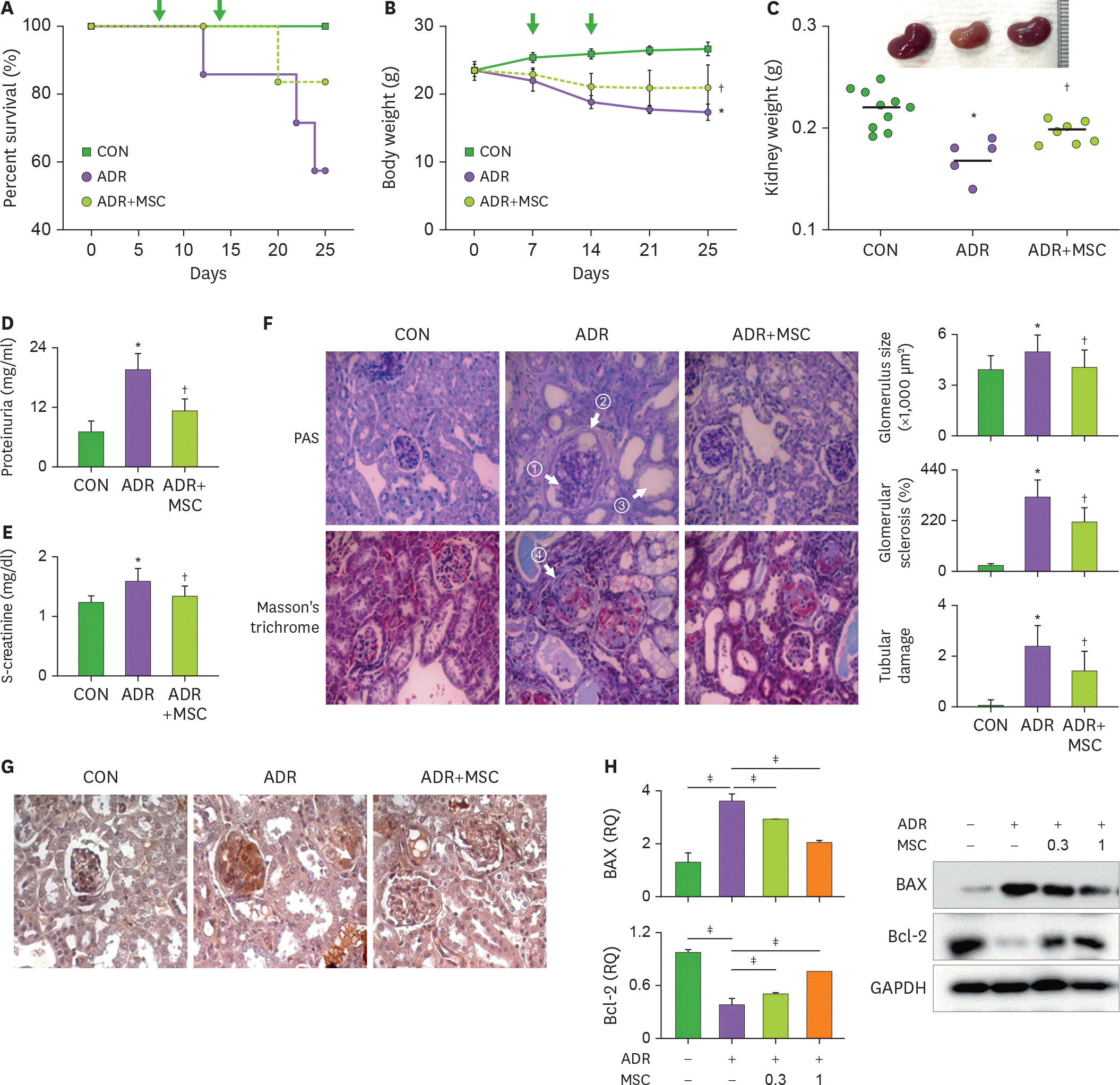
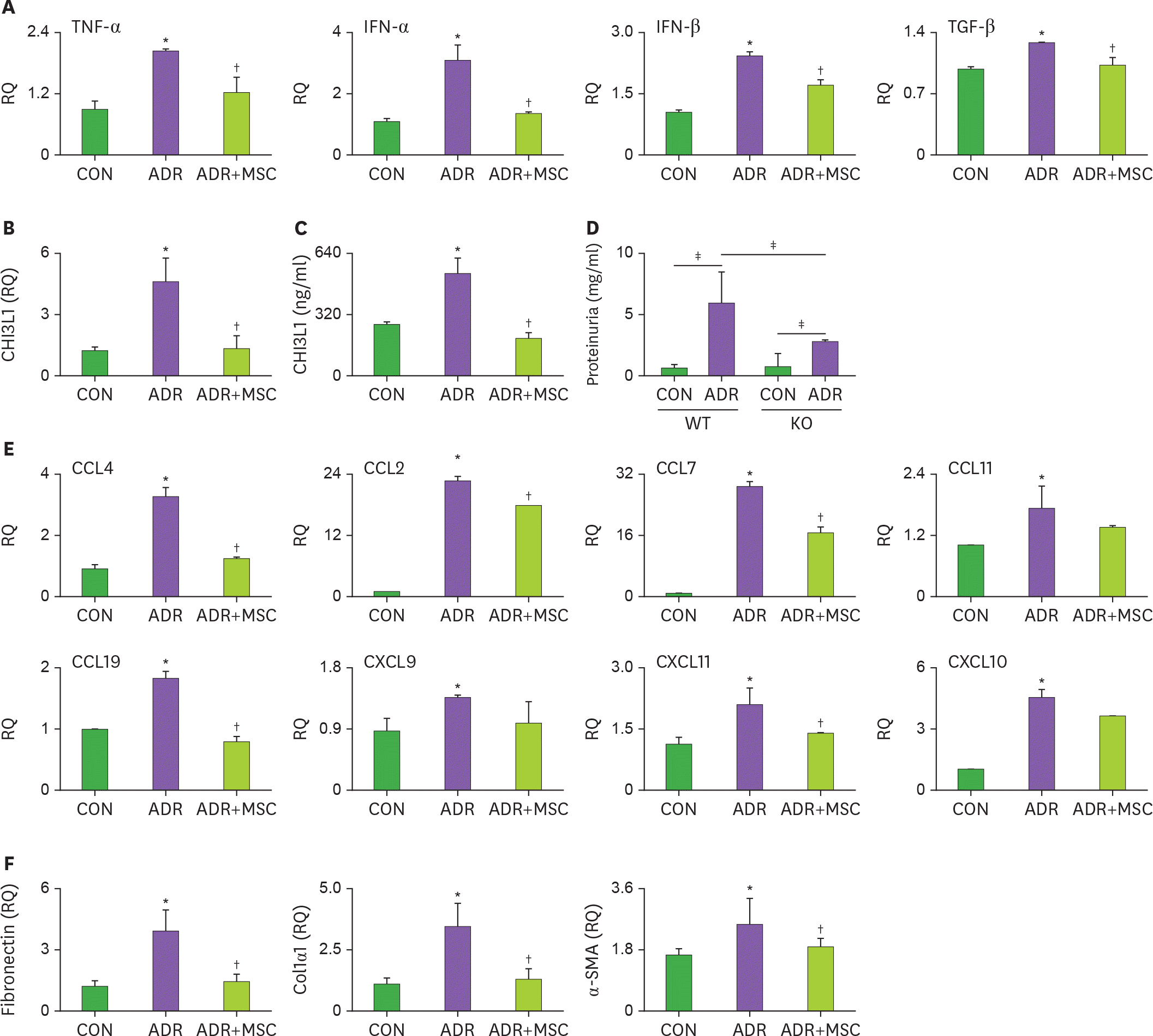
Figure 2.
MSCs inhibit renal inflammation in ADR-treated mice. (A-C, E, and F) Male BALB/c mice were intravenously injected with 10 mg/kg of ADR on day 0, and MSCs (1×106 cells/mouse) on days 7 and 14. Mice were sacrificed on day 28. The amounts of mRNA of cytokines (A), CHI3L1 (B), chemokines (E), and extracellular proteins, such as fibronectin, Col1α1, and α-SMA (F) were measured by RT-qPCR. (C) The level of CHI3L1 in serum was measured by ELISA. Each group are presented as CON (chemically-untreated control mice; n=6), ADR (ADR-treated mice; n=6), and ADR+MSC (ADR and MSC-treated mice; n=7), respectively. (D) ADR was injected to WT male C57BL/6 or CHI3L1 KO mice (C57BL/6 background) on day 0 and proteinuria level was measured on day 28. Each group are presented as CON (chemically-untreated control mice; n=4) and ADR (ADR-treated mice; n=4), respectively. * p<0.05 CON versus ADR; † p<0.05 ADR versus ADR+MSC; ‡ p<0.05.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download