This article has been
cited by other articles in ScienceCentral.
Abstract
Neutrophilic granule protein (NGP) was previously reported as a granular protein of neutrophils in mouse, but the function has not been known clearly. We found the presence of the possible signal peptide in NGP and validated this protein is circulating in the bloodstream. In our findings, NGP is being modified post-translationally in Golgi apparatus and endoplasmic reticulum, which is a universal character of secretory molecules with a signal peptide. The secreted NGP protein could be detected both in vitro and in vivo. NGP has sequence similarity with an antimicrobial protein cathelicidin, and we observed the aspect of inflammation of NGP. Interestingly, NGP interacts with the complex of LPS and LPS binding protein (LBP). This interaction blocks the binding of the complex of LPS and LBP to TLR4 and the downstream inflammatory signals. Furthermore, the inhibitory function of NGP against the inflammatory effect of LPS could be observed in both in vitro and in vivo. With these findings, we report NGP is a novel secretory protein to mask LPS and inhibit its function.
Keywords: Inflammation, Inhibitors, Lipopolysaccharides, Neutrophil, Protein binding, Cytokines
Abbreviations
magnetic-activated cell sorting
neutrophilic granule protein
INTRODUCTION
Leukocytes contain several antimicrobial proteins and peptides for the defense of the host organism that undertake through either specific and non-specific pathways. Among the best-studied are bactericidal/permeability-increasing protein (
12), the defensins (
3), azurocidin (CAP37) (
4), and LL-37 (
56).
LPS initiates signaling triggered by the immunological response to gram-negative bacteria. LPS from several different bacterial species or strains initiate acute inflammatory responses in the host that is a typical host reaction to infection and immune cell responses to LPS exposure. LPS can initiate a robust immune response and serves as an early warning signal of bacterial infection. LPS is first released from bacterial membranes and vesicles from them by LPS binding protein (LBP) in host serum. LBP then donates LPS to CD14, which can be found either in soluble form or linked to the cell surface by a GPI anchor. CD14 splits LPS complex into monomeric molecules and donates them to the TLR4–MD-2 complex. Aggregation of the TLR4–MD-2 complex after binding LPS leads to activation of further signaling components, including NF-κB and IFN regulatory factor 3, and followed by the production of pro-inflammatory cytokines (
7891011).
Neutrophilic granule protein (NGP) was initially identified in immature bone marrow (BM) cells and promyelocytes. This 19-kDa myeloid granule protein shows 30% homologous to cathelicidin, which is a member of cystatin superfamily (
12). It has been reported that NGP gene expression is controlled by C/EBP-ε and PU.1 transcription factors, which might be like other myeloid genes (
13).
Which cells express NGP and how this protein is transcribed are revealed. However, the molecular function of NGP protein in vitro and in vivo remains still unclear. In accordance with the sequence homology with antimicrobial peptide, cathelicidin. We hypothesized NGP might have a role in LPS biology. In this study, we focused on the biochemical and functional characterization of NGP.
MATERIALS AND METHODS
Animals
The mice were maintained in a pathogen-free facility at the National Cancer Institute (Frederick, MD, USA) in accordance with Animal Care and Use Committee regulations (protocol number 17-009, 17-010 and 17-048). C57BL/6J mice were purchased from the Jackson Lab, and NGP deficient mice on the C57/BL6 background were purchased from Knockout Mouse Project Repository (KOMP, Davis, CA, USA) and backcrossed to C57BL/6J. Sex and age-matched mice were used in all the studies. The left anterior descending artery-ligation was performed as described previously (
14). For the LPS injection, 0.2 mg/kg of LPS was prepared in saline solution and injected intraperitoneally.
Cell culture and primary cell isolation
EL-4, Raw 264.7, 293T, THP-1, and HL-60 cell lines were obtained from American Type Culture Collection and maintained according to the instructions. Mouse blood cells were sorted from the total blood or BM of mice by magnetic-activated cell sorting (MACS; #130-110-434, #130-100-629, #130-097-658, Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer description. For BM cell isolation, we ground fresh long bones from legs and homogenized to maximize the yield. Next, we isolated neutrophils from the homogenized cells by MACS (Miltenyi Biotec). For the overexpression and short hairpin RNA (shRNA), lentivirus of pHage-NGP and NGP shRNA were prepared in 293T cells following the co-transfection of envelope vector, pMD2g, and packaging vector, psPAX2. The prepared lentivirus was transduced to cells by polybrene. Mouse neutrophil was isolated from the peritoneal cavity 3 h after injection of 1 ml thioglycollate medium (Sigma-Aldrich, St. Louis, MO, USA). Isolated neutrophils were incubated at 37°C for 6 h and then treated with 10 ng/ml, 100 ng/ml, 1μg/ml, and 10 μg/ml LPS overnight. NGP conditional medium was collected 2 days after the transfection of NGP to 293T cells by Fugene HD (Promega, Madison, WI, USA). 10 ng/ml of LPS was mixed with the conditional medium for 30 min and then treated to THP-1 cells.
Quantitative RT-PCR (qRT-PCR)
qRT-PCR was performed using total RNA isolated on RNeasy Quick spin columns (QIAGEN, Valencia CA, USA). One μg of total RNA was used to perform RT-PCR using iScript supermix (Biorad, Hercules, CA, USA). The sequence of PCR primers used is NGP, 5′-AGACCTTTGTATTGGTGGTGGC-3′ for sense and 5′-GGTTGTATGCCTCTATGGCTCTA-3′ for reverse; β-actin, 5′-CTGTCCCTGTATGCCTCTG-3′ for sense and 5′-ATGTCACGCACGATTTCC-3′ for reverse. Values are expressed as fold increase relative to β-actin and analyzed with CFX Manager (Biorad). All primers were purchased from Sigma-Aldrich.
Generation of NGP Ab
The cDNA sequence of the mature NGP was cloned to pProEx/HT (Thermo Fisher Scientific, Waltham, MA, USA) vector. NGP construct was transformed to BL21-Codon plus (Agilent, Santa Clara, CA, USA) and induced with 6 mM IPTG for 3 h. The expressed protein was purified by Talon metal affinity chromatography (TAKARA, Kusatsu, Japan) following the description (Cat. #635606). The purified recombinant protein was used as the Ag and immunized to rabbits. The immunization and preparation of the Ab were produced by Rockland (Limerick, PA, USA), following Rockland/NCI MTA protocol. The Ab is listed in the lineup of Rockland (Cat. # 600-401-GW9). The quality of Ab was tested by ELISA and Western blot (
Supplementary Fig. 1).
Immunoprecipitation and Western blot
The conditional medium of NGP expressing THP-1 cells, HL-60, or 293T cells, were used for immunoprecipitation by Myc Ab (Cell Signaling, Danvers, MA, USA). LPS, LBP, and the NGP conditional medium were mixed at 4°C for 30 min, and then NGP was pulled down with Myc Ab. The IP product was propagated to Western blotting for Myc tagged NGP detection. The total product of IP was propagated for Western blot using Myc Ab. NGP overexpressing THP-1 cells were lysed by a 1× sample buffer, and then Western blotting was performed by Myc Ab (Cell Signaling). For NGP Western blot, total lysate of mouse cells, mouse tissues, serum, or peritoneal neutrophils were used and probed by NGP Ab we generated. IκBα and p-p38, p38 were detected from LPS treated THP-1 cells with or without NGP conditional medium. The Abs were from Cell Signaling.
Immunostaining and confocal microscopy
THP-1 cells and HL-60 cells were transduced with NGP-Myc lentivirus. Cells were fixed with paraformaldehyde and stained against Golga3, Calreticulin, and NGP. Golgia3 and Calreticulin Abs were obtained from Abcam. Confocal microscopy was performed with LSM-780 (Zeiss, Oberkochen, Germany) and analyzed with ImageJ (NIH, Bethesda, MD, USA).
Sandwich and direct ELISA
Mouse TNFα, IL-1β, and IL-6 were detected by ELISA duo sets (R&D Systems, Minneapolis, MN, USA) following the manual. For direct ELISA of LPS receptors, recombinant MD2, CD14, or MD2/TLR4 complex was coated on an ELISA plate overnight at 1 μg/ml concentration. The plate was blocked by 5% BSA containing PBS and mixture of 100 ng/ml of biotinylated LPS, 100 ng/ml of recombinant LBP, and the conditional medium of NGP or empty vector from 293T cells for 2 h. Biotinylated LBP was detected by TMB solution (Sigma-Aldrich).
Molecular cloning
Reporter assay
the pGluc-NF-κB construct was obtained from Genecopoeia (Rockville, MD, USA). pSV40-CLuc control plasmid was obtained from NEB (Ipswich, MA, USA). These constructs were co-transfected to 293 cells by FugeneHD (Promega). Transfected cells were attached in a 96-well plate, and then 0, 1, 10, or 100 ng/ml of LPS was treated overnight. The culture supernatant was projected to perform luciferase assay with Gaussia Luciferase assay kit and Cypridina Luciferase assay kit (NEB) following their description.
Statistical analysis
All statistical analyses were carried out using Prism 7 (La Jolla, CA, USA). Quantitative variables were analyzed by paired Student's t-test and 2-way ANOVA test with multiple comparisons. All statistical analysis was 2-sided, and p<0.05 was considered statistically significant.
RESULTS
Neutrophils express NGP
First, we validated the expression of NGP in the mouse. Total RNA of T cells, B cells, macrophages, monocytes, BM-derived neutrophils, and bloodstream neutrophils were isolated. We compared the mRNA levels of NGP in mouse cells and BM neutrophils, and blood neutrophils had a high expression of NGP, while macrophages and monocytes had lower expression. T cells, B cells, and NGP deficient neutrophils had no expression of NGP in mRNA (
Fig. 1A). Next, we also verified the protein expression in mouse neutrophils. We collaborated with Rockland to generate NGP Ab because NGP Abs are not commercially available. We confirmed the expression of NGP from mouse neutrophils by Western blot while EL4 mouse T cell line, Raw 264.7 mouse macrophage cell line, and NGP deficient neutrophils had no expression of NGP protein (
Fig. 1B). The expression of NGP protein was specific in hematopoietic tissue, including neutrophils like BM. This protein was not expressed in other tissues such as lung, heart, stomach, ileum, colon, liver, kidney spleen, and thymus (
Fig. 1C).
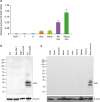 | Figure 1NGP is expressed specifically in mouse neutrophils. (A) T cells, B cells, macrophages, monocytes, BM-derived neutrophils, and circulating neutrophils were isolated from a WT mouse. mRNA level of NGP was relatively measured by qRT-PCR and compared to NGP deficient neutrophils as the negative control and blood neutrophil as the positive control (n=3, quadruplet mean±SD). (B) NGP protein was detected by Western blotting from the total lysate of EL4 cells, Raw 264.7 cells, NGP deficient neutrophils, and WT neutrophils by Western blot. (C) NGP protein was detected by Western blotting from the total lysate of mouse tissues. Lung, heart, stomach, ileum, colon, liver, kidney, spleen, thymus, and BM were collected from a WT mouse. Tissue was ground in 1× sample buffer, and NGP was detected by Western blotting.
|
NGP is a soluble secretory molecule
The protein sequence on NGP is anticipated to have a signal peptide with typical 19 hydrophobic amino acids (
Fig. 2A). We hypothesized this protein is simultaneously secreted from cells. NGP was overexpressed in THP-1 and HL-60 cells that have granules and 293T cells to observe the secretion of NGP. The 2-day culture medium of these cells had NGP proteins in the medium without any special stimulation (
Fig. 2B). Interestingly the location of NGP was endoplasmic reticulum (ER) than granular structures in HL60 cell and THP-1 cells overexpressing NGP (
Fig. 2C). In addition, when NGP overexpressing THP-1 cells were stimulated by brefeldin A (BFA) or monensin to block the function of ER and Golgi consequently, NGP protein in the cell was increased. Furthermore, when post-translational modification in the ER was blocked by BFA, NGP protein had a smaller molecular size than from vehicle or monensin treatment (
Fig. 2D). The location of endogenous NGP in peritoneal neutrophils was in ER-like NGP was overexpressed (
Fig. 2E). When peritoneal neutrophils were stimulated by BFA or monensin, NGP protein in the cell was increased, and the secretion of NGP was reduced (
Fig. 2F). Unlike in overexpressed cells (
Fig. 2D), the intracellular NGP was relatively lower in peritoneal neutrophils (
Fig. 2F).
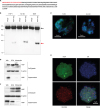 | Figure 2
NGP is a soluble secretory molecule. (A) The amino acid sequence of NGP. Red letters stand for the area of the signal peptide (red). (B) Myc-tagged NGP was pulled down from NGP overexpressed THP-1, HL-60, or 293T cells. Myc-tag was detected by Western blotting, and the red arrow shows NGP-Myc band. The black arrow shows IgG heavy chain. Representative images are shown. (C) NGP overexpressing HL-60 (upper panel), and THP-1 (lower panel) were stained against Golga3 (red), Calreticulin (green), and NGP (blue). The stained cells were analyzed by confocal microscopy. (D) NGP-Myc overexpressing THP-1 cells were treated by veh, 1× BFA, or 1× monensin (Biolegend) for 3 h. Myc-tagged NGP was detected from the total lysate and supernatant by Western blotting. A representative result out of 3 experiments is shown. (E) Mouse peritoneal neutrophils were stained against NGP (green), Calreticulin (blue), and lysobrite for granules (red). The stained cells were analyzed by confocal microscopy. A representative image is shown. (F) Mouse peritoneal neutrophils were treated by veh, 1× BFA, or 1× monensin (Biolegend) for 3 h. NGP was detected from the total lysate and supernatant by Western blotting. A representative result out of 3 experiments is shown.
Veh, vehicle.

|
NGP is induced by LPS stimulation
Neutrophils were isolated from mouse peritoneal cavity, and 100 ng/ml or 500 ng/ml of LPS was treated for 6 h to these cells. We detected the expression of NGP protein by Western blot, and observed NGP protein level is increased when mouse neutrophil is stimulated by LPS (
Fig. 3A). Next, we isolated mouse serum after stimulation with LPS. NGP protein was detected in the mouse serum 3 h after mice were stimulated with LPS, while NGP deficient mouse did not show any NGP protein in the serum (
Fig. 3B). The function of secreted NGP in inflammation was assessed from the mouse serum. We injected 0.2 mg/kg of LPS and collected mouse blood to isolate mouse serum. The collected serum was propagated to perform ELISA of TNFα, IL-1β, or IL-6. The levels of all TNFα, IL-1β, and IL-6 were increased when NGP was knocked out in mice (
Fig. 3C).
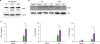 | Figure 3
NGP is induced by LPS. (A) Mouse peritoneal neutrophils were treated by vehicle, 100 ng/ml, or 500 ng/ml of LPS for 3 h. NGP was detected from the total lysate by Western blotting. (B) 1 mg/kg of LPS was injected into WT mouse or NGP deficient mouse intraperitoneally. The serum was isolated from each animal 5 h after injection. NGP was probed from the serum by Western blotting. (C) 0.2 mg/ml of LPS was injected into WT (black bar) or NGP deficient (empty bar) mice. Mouse serum was collected before injection of LPS, 1 h after injection for detection of IL-1β and IL-6, and 3 h after injection to detect TNFα. TNFα, IL-1β, and IL-6 were detected by ELISA (quadruplet mean±SD). Each experiment was repeated 3 times, and representative data is selected.
*p<0.05, ***p<0.001.

|
NGP interacts with LBP
The next question was how NGP inhibits the LPS function. We mixed LPS and/or LBP in the conditional medium of empty vector or Myc-tagged NGP from 293T cells. Then we pulled down NGP with the tag Ab. NGP was pulled down from the overexpressed conditional medium, and interestingly, LBP protein was pulled down with NGP only in the presence of LPS. This shows NGP pulls down LBP only when LPS exists (
Fig. 4A). We prepared wild type (WT) and 5 different NGP mutant clones with C-terminal Myc-tag to identify the binding site of LPS-LBP complex and NGP (
Fig. 4B). The culture medium of WT or NGP mutants were mixed with LBP and LPS, and then NGP was pulled down with Myc tag. WT NGP and C-terminal 20, 60, and 90 amino acid deletion mutants pulled down LBP (
Fig. 4C); however, the deletion mutant of Glu20-Leu47 did not pull down LBP unlike WT NGP or other mutants (
Fig. 4D). Next, we screened which of LPS receptors is being blocked for LPS-LBP complex to bind. We coated recombinant MD2, CD14 TLR4, or TLR4/MD2 complex in the ELISA plate and added the mixture of LPS and NGP in control or NGP conditional medium. LPS binding to the coated receptor component was inhibited with NGP conditional medium when TLP4 or TLR4/MD2 complex was coated, unlike when MD2 or CD14 was coated, which shows NGP inhibits the binding of LPS-LBP complex to TLR4 (
Fig. 4E).
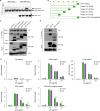 | Figure 4
NGP masks LPS and inhibits LPS receptor binding. (A) Conditional medium of NGP or empty vector was mixed with 100 ng of recombinant LBP-His and/or 100 ng of LPS for 30 min on ice. LBP or NGP was detected by Western blotting. (B) Five different NGP mutant constructs were prepared to identify the binding site of LPS-LBP complex on NGP. Amino acids are deleted from the C-terminus in mutant 1–4 as designated. Mutant 5 has a deletion of 28 amino acids after signal peptide. (C and D) WT NGP, or deletion mutants of NGP were mixed with 100 ng of LPS and 100 ng of LBP for 30 min on ice. NGP was pulled down with Myc-tagged NGP, and LBP and Myc-tagged NGP were detected by Western blotting from both IP products and input. (E) Recombinant MD2, CD14, TLR4, or TLR4/MD2 complex was coated on an ELISA plate. Biotinylated LPS was mixed with 100 ng of LBP in designated concentration for 30 min on ice. The mixture of LPS and LBP was added to the receptor coated plate for 2 h. Bound LPS was visualized by TMB solution and analyzed by ELISA reader (quadruplet mean±SD).
GFP, green fluorescent protein.
**p<0.01.

|
NGP inhibits LPS activity
We transfected NGP or empty vector in 293T cells to collect the conditional medium of NGP 2 days post-transfection. We mixed conditional medium of empty vector and NGP vector in several ratios and then added this medium to THP-1 cells. Then we treated 10 ng/ml of LPS to THP-1 cells and incubated overnight. We harvested the LPS treated medium and measured IL-8 secretion by ELISA. IL-8 was highly secreted in the empty vector conditional medium, but it was remarkably decreased when NGP conditional medium was mixed in high concentration. This suppression of IL-8 was lower as the concentration of NGP conditional medium was low (
Fig. 5A). We further assessed whether the LPS mediated downstream signals are affected by NGP treatment. The conditional medium of the empty vector or NGP vector was mixed with vehicle or LPS and then treated to THP-1 cells. As a result, LPS induced the degradation of IκBα in the cell within the empty vector medium while NGP medium kept the level of IκBα high and decreased the phosphorylation of NF-κB p65 (
Fig. 5B). In similarly way, the phosphorylation of p38 MAPK was increased high when LPS was treated in an empty vector medium, but the phosphorylation was lowered when cells were in NGP conditional medium (
Fig. 5C). The conditional medium of NGP inhibited the activity of LPS mediated induction of NF-κΒ. LPS was mixed in different concentrations and then treated to 293 cells transfected with Gaussia luciferase NF-κB construct. The luminescence of cells with NGP medium showed inhibited activity of the reporter (
Fig. 5D). Next, we collected mouse peritoneal neutrophils from WT or NGP deficient mice. We treated LPS to these neutrophils in different concentrations overnight and then collected culture supernatant. The level of inflammatory cytokine, IL-6 was measured by ELISA. The level of secreted IL-6 increased as the concentration of LPS was higher, and IL-6 was secreted much more when the neutrophils were collected from NGP deficient mice (
Supplementary Fig. 2).
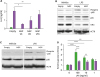 | Figure 5
NGP blocks the activity of LPS. (A) THP-1 cells were treated by vehicle (black bars) or 100 ng/ml of LPS (blank bars) for 16 h in the mixture of NGP conditional medium and empty vector conditional medium in a ratio of 0, 1/4, 1/8, and 1/16. IL-8 was detected from the culture medium (quadruplet mean±SD). (B) THP-1 cells were treated by vehicle or 10 ng/ml of LPS in an empty vector or NGP conditional medium for 30 min. Phospho-NF-κB p65, NF-κB p65, and IκBα were detected by Western blotting. (C) THP-1 cells were treated by vehicle or 10 ng/ml of LPS in an empty vector or NGP conditional medium for 30 min. Phospho-p38 MAPK and p38 MAPK were detected by Western blotting. (D) The conditional medium of empty vector (black) or NGP (blank) was treated overnight. LPS was mixed in different concentrations and then treated to 293 cells transfected with Gaussia luciferase NF-κB construct. The luminescence of cells with NGP medium showed inhibited activity of the reporter (quadruplet mean±SD).
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.

|
DISCUSSION
NGP was first discovered in mouse neutrophils, and the function has not been known. The closest orthologue in humans is cathelicidin, which is an antimicrobial protein, and we hypothesized this protein has a role in bacterial infection. We first validated this molecule is expressed in mouse neutrophils. Several lineages of mouse blood cells were isolated and assessed the expression of NGP mRNA in those cells. As it has been known, the level of NGP was solely high in BM or circulating neutrophils, and the level was higher in circulating neutrophils than BM neutrophils.
We had a limitation to study NGP molecule because the Ab against NGP is not commercially available. The Ab against NGP had to be generated, so we expressed and purified recombinant NGP protein from Escherichia coli. This recombinant protein was used as the Ag to generate NGP Ab from a rabbit in collaboration with Rockland. Through the validation of the Ab by Ag-direct ELISA and Western blot using the Ag, the Ab showed high specificity and affinity to both recombinant and endogenous NGP protein. We could detect NGP protein in mouse neutrophils, finally using this NGP Ab. As we expected, NGP protein could be easily detected from mouse neutrophils, unlike other mouse leukocyte cell lines or NGP deficient mouse neutrophils. Furthermore, NGP expression could be detected from BM, which has a high portion of PMN cells than other mouse organs like lung, heart, stomach, intestine, liver, kidney, spleen, and thymus. This confirmed NGP protein is neutrophil specific protein. Interestingly, NGP protein had a double band under Western blot, which may be the result of post-translational protein that is vigorously modified and secreted.
The amino acid sequence of NGP protein has typical 19 hydrophobic amino acids on its N-terminal, so we hypothesized this protein is being secreted out of cells. We validated the secreted NGP protein in the culture medium when NGP is overexpressed from THP-1 cells and HL-60 cells that have granules and in 293T cells. Next, we overexpressed NGP in THP-1 cells and blocked the protein maturation in the ER or Golgi with BFA or monensin consequently. When these steps were blocked, the protein level of NGP was kept high in the cell, not being secreted. Furthermore, the molecular size of NGP was lower when the ER process was blocked, which showed this protein is being modified in ER, which also is classic evidence this protein goes through secretory protein processing. The location and the secretory characters that we found from the overexpressed cells could also be observed from endogenous mouse peritoneal neutrophils. Meanwhile, the intracellular NGP of peritoneal neutrophils was remarkably lower than that of NGP-overexpressed THP-1 cells. The constitutively active overexpression of NGP under cytomegalovirus promoter may be the reason for this discrepancy.
We also wondered if this protein is being secreted into the bloodstream and whether this protein is being affected by bacterial stimulation, such as LPS treatment in vivo. We first observed the protein level of NGP was increased when primary mouse neutrophil was stimulated by LPS in a dose-dependent manner. Next, the protein in the circulating mouse blood could be detected in the isolated serum of mice when mice got an injection of LPS. NGP is secreted into the bloodstream in response to LPS, and then we wondered whether NGP affects any function of LPS. Interestingly, the increase of pro-inflammatory cytokines such as TNFα, IL-1β, and IL-6 in NGP deficient mice by the stimulation with LPS was much higher than WT mice. This showed NGP is secreted into the bloodstream by LPS stimulation and lowers the inflammatory effect of LPS.
The inhibition of LPS mediated pro-inflammatory cytokine activation was also inhibited when the conditional medium of NGP was mixed with LPS in vitro. Logically the downstream signaling like p38 MAPK and NF-κB by LPS stimulation on CD14-TLR4-MD2 pathway was downregulated when NGP was upregulated in the cell. Furthermore, the isolated neutrophils of NGP deficient mice showed a much more sensitive response to LPS, unlike WT mice. This shows NGP is an inhibitor of LPS not only in vivo but also in vitro.
The binding of NGP to the LPS-LBP complex is the key mechanism to suppress LPS activity. NGP binds to LBP in the presence of LPS. NGP did not bind to LBP when LPS was not mixed with LPS. We wondered which site of NGP binds to the LPS-LBP complex, so we prepared several different deletion mutants of NGP protein. This revealed amino acids between Glu20-Leu47 of NGP bind to the LBP-LPS complex. In addition, the interaction of the LPS-LBP complex with the LPS receptor could be inhibited by NGP. A component of LPS receptor, TLR4, binds to LPS-LBP complexes like other components, MD2, and CD14 in a dose-dependent manner, but when the LPS-LBP complex is mixed with NGP conditional medium, the interaction of TLR4 with LPS-LBP complex was significantly inhibited. This shows NGP is an antagonist of LPS-LBP complex and inhibits the interaction of this complex to TLR4 (
Fig. 6).
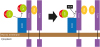 | Figure 6 NGP masks LPS-LBP complex. The complex of LPS and LBP stimulates the LPS receptor complex of CD14, TLR4, and MD2. NGP binds to the LPS-LBP complex and masks the complex to interact with its receptor. LPS-LBP complex fails to bind to TLR4 from CD14 and followed by the null downstream signal of TLR4.
|
In this study, we found the signal peptide sequence of NGP molecule that secretes this protein from cells. NGP captures LPS-LBP complex and inhibits the interaction with TLR4, which blocks the downstream activation of the TLR4 pathway. NGP is not the only inhibitor of LPS, but this molecule blocks the function of LPS at the highest level of LPS signaling. In history, the fight of the host immune system against bacterial infection has always been challenging and will be continued forever. NGP may not enough to overcome endotoxemia or endotoxin shock of the host; however, NGP gives us a direction to cure an additive or substitutive therapy of bacterial infection.
Abbreviations
magnetic-activated cell sorting
neutrophilic granule protein
ACKNOWLEDGEMENTS
Hong J, Wuest TR, and Lin PC are supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
1. Gazzano-Santoro H, Parent JB, Grinna L, Horwitz A, Parsons T, Theofan G, Elsbach P, Weiss J, Conlon PJ. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992; 60:4754–4761.

2. Shafer WM, Martin LE, Spitznagel JK. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984; 45:29–35.

3. Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993; 11:105–128.

4. Pereira HA, Erdem I, Pohl J, Spitznagel JK. Synthetic bactericidal peptide based on CAP37: a 37-kDa human neutrophil granule-associated cationic antimicrobial protein chemotactic for monocytes. Proc Natl Acad Sci U S A. 1993; 90:4733–4737.

5. Hirata M, Yoshida M, Inada K, Kirikae T. Investigation of endotoxin binding cationic proteins from granulocytes; agglutination of erythrocytes sensitized with Re-LPS. Adv Exp Med Biol. 1990; 256:287–299.

6. Larrick JW, Hirata M, Shimomoura Y, Yoshida M, Zheng H, Zhong J, Wright SC. Antimicrobial activity of rabbit CAP18-derived peptides. Antimicrob Agents Chemother. 1993; 37:2534–2539.

7. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004; 4:499–511.

8. Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999; 274:10689–10692.

9. Cohen J. The immunopathogenesis of sepsis. Nature. 2002; 420:885–891.

10. Pugin J, Schürer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993; 90:2744–2748.

11. Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002; 71:635–700.

12. Moscinski LC, Hill B. Molecular cloning of a novel myeloid granule protein. J Cell Biochem. 1995; 59:431–442.

13. Gombart AF, Kwok SH, Anderson KL, Yamaguchi Y, Torbett BE, Koeffler HP. Regulation of neutrophil and eosinophil secondary granule gene expression by transcription factors C/EBP epsilon and PU.1. Blood. 2003; 101:3265–3273.

14. Huang J, Lv G, Min Y, Yang L, Lin PC. Intravenous administration of Gr-1+CD11b+ myeloid cells increases neovascularization and improves cardiac function after heart infarction. Int J Cardiol. 2013; 168:1702–1705.

SUPPLEMENTARY MATERIALS
Supplementary Figure 1
(A) 1 μg/ml of recombinant NGP was coated on an ELISA plate. Preimmune serum and immuned serum of NGP from the rabbit was added in designated concentration for 2 h at room temperature. The bound Ab was measured by donkey anti-rabbit IgG-horseradish peroxidase Ab and visualized by TMB solution. The signal was analyzed by ELISA reader. (B) 1 ng or 10 ng or recombinant NGP was detected by Western blotting with NGP immuned serum or preimmune serum.
Supplementary Figure 2
Peritoneal neutrophils of a WT mouse or NGP deficient mouse were isolated and stabilized in 10% FBS containing RPMI for 6 hours. LPS of designated concentrations was treated overnight to neutrophils. IL-6 was detected from the culture medium by ELISA (quadruplet mean±SD).










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download