1. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015; 74(1):65–73.

2. Fabiani C, Vitale A, Orlando I, Capozzoli M, Fusco F, Rana F, et al. Impact of uveitis on quality of life: a prospective study from a tertiary referral rheumatology-ophthalmology collaborative uveitis center in Italy. Isr Med Assoc J. 2017; 19(8):478–483.
3. Maxwell LJ, Zochling J, Boonen A, Singh JA, Veras MM, Tanjong Ghogomu E, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015; (4):CD005468.

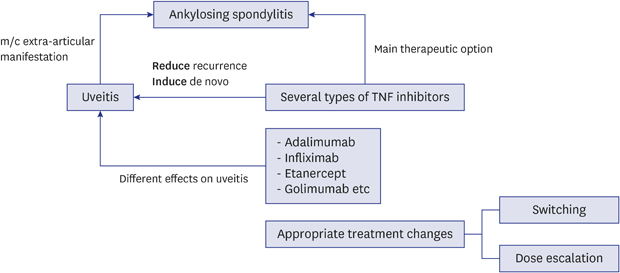
4. Braun J, Baraliakos X, Listing J, Sieper J. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum. 2005; 52(8):2447–2451.

5. Rudwaleit M, Rødevand E, Holck P, Vanhoof J, Kron M, Kary S, et al. Adalimumab effectively reduces the rate of anterior uveitis flares in patients with active ankylosing spondylitis: results of a prospective open-label study. Ann Rheum Dis. 2009; 68(5):696–701.

6. Kim M, Won JY, Choi SY, Ju JH, Park YH. Anti-TNFα treatment for HLA-B27-positive ankylosing spondylitis-related uveitis. Am J Ophthalmol. 2016; 170:32–40.

7. De Vos AF, Van Haren MA, Verhagen C, Hoekzema R, Kijlstra A. Systemic anti-tumor necrosis factor antibody treatment exacerbates endotoxin-induced uveitis in the rat. Exp Eye Res. 1995; 61(6):667–675.

8. Kakkassery V, Mergler S, Pleyer U. Anti-TNF-alpha treatment: a possible promoter in endogenous uveitis? observational report on six patients: occurrence of uveitis following etanercept treatment. Curr Eye Res. 2010; 35(8):751–756.
9. Wendling D, Paccou J, Berthelot JM, Flipo RM, Guillaume-Czitrom S, Prati C, et al. CRI. New onset of uveitis during anti-tumor necrosis factor treatment for rheumatic diseases. Semin Arthritis Rheum. 2011; 41(3):503–510.

10. Guignard S, Gossec L, Salliot C, Ruyssen-Witrand A, Luc M, Duclos M, et al. Efficacy of tumour necrosis factor blockers in reducing uveitis flares in patients with spondylarthropathy: a retrospective study. Ann Rheum Dis. 2006; 65(12):1631–1634.

11. Wendling D, Joshi A, Reilly P, Jalundhwala YJ, Mittal M, Bao Y. Comparing the risk of developing uveitis in patients initiating anti-tumor necrosis factor therapy for ankylosing spondylitis: an analysis of a large US claims database. Curr Med Res Opin. 2014; 30(12):2515–2521.

12. Fabiani C, Vitale A, Lopalco G, Iannone F, Frediani B, Cantarini L. Different roles of TNF inhibitors in acute anterior uveitis associated with ankylosing spondylitis: state of the art. Clin Rheumatol. 2016; 35(11):2631–2638.

13. Lie E, Lindström U, Zverkova-Sandström T, Olsen IC, Forsblad-d'Elia H, Askling J, et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Ann Rheum Dis. 2017; 76(9):1515–1521.

14. Reddy AR, Backhouse OC. Does etanercept induce uveitis? Br J Ophthalmol. 2003; 87(7):925.

15. Raffeiner B, Ometto F, Bernardi L, Botsios C, Punzi L. Inefficacy or paradoxical effect? Uveitis in ankylosing spondylitis treated with etanercept. Case Rep Med. 2014; 2014:471319.

16. van Bentum RE, Heslinga SC, Nurmohamed MT, Gerards AH, Griep EN, Koehorst CB, et al. Reduced occurrence rate of acute anterior uveitis in ankylosing spondylitis treated with golimumab: the GO-EASY study. J Rheumatol. 2019; 46(2):153–159.
17. Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, et al. Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn's disease. Gastroenterology. 2003; 124(7):1774–1785.

18. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30(2):239–245.

19. Gulyas K, Bodnar N, Nagy Z, Szamosi S, Horvath A, Vancsa A, et al. Real-life experience with switching TNF-α inhibitors in ankylosing spondylitis. Eur J Health Econ. 2014; 15(Suppl 1):S93–100.

20. Deodhar A, Yu D. Switching tumor necrosis factor inhibitors in the treatment of axial spondyloarthritis. Semin Arthritis Rheum. 2017; 47(3):343–350.

21. Dhingra N, Morgan J, Dick AD. Switching biologic agents for uveitis. Eye (Lond). 2009; 23(9):1868–1870.

22. Bandrés Ciga S, Salvatierra J, López-Sidro M, García-Sánchez A, Durán R, Vives F, et al. An examination of the mechanisms involved in secondary clinical failure to adalimumab or etanercept in inflammatory arthropathies. J Clin Rheumatol. 2015; 21(3):115–119.

23. Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011; 305(14):1460–1468.

24. Kneepkens EL, Wei JC, Nurmohamed MT, Yeo KJ, Chen CY, van der Horst-Bruinsma IE, et al. Immunogenicity, adalimumab levels and clinical response in ankylosing spondylitis patients during 24 weeks of follow-up. Ann Rheum Dis. 2015; 74(2):396–401.

25. Cordero-Coma M, Calleja-Antolín S, Garzo-García I, Nuñez-Garnés AM, Álvarez-Castro C, Franco-Benito M, et al. Adalimumab for treatment of noninfectious uveitis: Immunogenicity and clinical relevance of measuring serum drug levels and antidrug antibodies. Ophthalmology. 2016; 123(12):2618–2625.
26. Qiu Y, Chen BL, Mao R, Zhang SH, He Y, Zeng ZR, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J Gastroenterol. 2017; 52(5):535–554.

27. Ariza-Ariza R, Navarro-Sarabia F, Hernández-Cruz B, Rodríguez-Arboleya L, Navarro-Compán V, Toyos J. Dose escalation of the anti-TNF-alpha agents in patients with rheumatoid arthritis. A systematic review. Rheumatology (Oxford). 2007; 46(3):529–532.









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download