INTRODUCTION
METHODS
Materials
Cell culture and drug treatment
Western blot analysis
Measurement of NO production
Animal studies
Measurement of endothelium-dependent vessel relaxation
Statistical analysis
Ethics statement
RESULTS
Telmisartan decreases NO production and p-eNOS-Ser1179 levels in a time- and dose-dependent manner in BAECs exposed to hyperglycemia
 | Fig. 1Tel reduces NO production and the phosphorylation of eNOS at Ser1179 in a time- and dose-dependent manner in hyperglycemic BAECs. (A) NO production was measured as nitrite (a stable metabolite of NO) in cell culture supernatants after BAECs were treated with various concentrations of Tel (0, 5, 10, or 20 μM) for 24 hours in the presence of 25 mM D-glucose or D-mannitol as described in the METHODS. (B) BAECs were treated as described above, and then p-eNOS-Ser1179 levels were assessed by western blotting. Nitrocellulose membranes were re-probed with anti-eNOS antibody to confirm equal sample loadings. (C) BAECs were treated with vehicle (DMSO) or 20 μM Tel for various times (1, 2, 4, 8, or 24 hours) in the presence of 25 mM D-glucose, and then NO production was measured as described in Fig. 1A. (D) BAECs were treated as described above, and p-eNOS-Ser1179 levels were assessed by western blotting. Nitrocellulose membranes were re-probed with anti-eNOS antibody to confirm equal sample loadings. Densitometry was used to quantitate p-eNOS-Ser1179 levels relative to total eNOS levels. All experiments were performed at least four times independently and the blots shown are representative of at least four experiments (n = 4). Bar graphs depict mean fold alterations below the controls (± standard deviation).Tel = telmisartan, NO = nitric oxide, eNOS = endothelial nitric oxide synthase, BAEC = bovine aortic endothelial cell, DMSO = dimethyl sulfoxide.
Differences were considered statistically significant at *P < 0.05, **P < 0.01, #P < 0.05, or ##P < 0.01.
|
Telmisartan increases PP2Ac expression, which mediates eNOS-Ser1179 dephosphorylation and consequently reduced NO production
 | Fig. 2Tel increases PP2Ac expression, which mediates eNOS-Ser1179 dephosphorylation and consequently reduced NO production. (A) BAECs were treated with various concentrations of Tel (0, 5, 10, or 20 μM) for 24 hours in the presence of 25 mM D-glucose, and then PP2Ac expression levels were assessed by western blotting. Nitrocellulose membranes were re-probed with anti-β-actin antibody to confirm equal sample loading. (B) BAECs were treated with vehicle (DMSO) or 20 μM Tel for various times (1, 2, 4, 8, or 24 hours) in the presence of 25 mM D-glucose, and then PP2Ac expression levels were detected by western blotting as described above. (C) BAECs were treated with 20 μM Tel for 24 hours in the presence of 25 mM D-glucose in the absence or presence of 5 nM okadaic acid, and then p-eNOS-Ser1179 levels were assessed by western blotting as described in Fig. 1B. (D) BAECs were treated as described above, and then NO production was measured as described in Fig. 1A. Densitometry was used to quantitate PP2Ac levels relative to β-actin or p-eNOS-Ser1179 levels relative to total eNOS. Experiments were performed at least four times independently and the blots shown are representative of at least four experiments (n = 4). Bar graphs depict mean fold alterations above the controls (± standard deviation).DMSO = dimethyl sulfoxide, Tel = telmisartan, Oka = okadaic acid, PP2Ac = protein phosphatase 2A catalytic subunit, eNOS = endothelial nitric oxide synthase, NO = nitric oxide, BAEC = bovine aortic endothelial cell.
Differences were considered statistically significant at *P < 0.05 or **P < 0.01.
|
Telmisartan is the only ARB to reduce p-eNOS-Ser1179 and NO levels, and to induce PP2Ac expression
 | Fig. 3Of the angiotensin II type 1 receptor blockers tested, only telmisartan decreases the phosphorylation of eNOS-Ser1179 and NO production and induces PP2Ac expression. (A, B) BAECs were treated with telmisartan, losartan, or fimasartan (all at 20 μM) for 24 hours in the presence of 25 mM D-glucose, and then levels of p-eNOS-Ser1179 and PP2Ac expression were assessed by western blotting, as described in Fig. 2. (C) BAECs were treated as described above, and then NO production was measured as described in Fig. 1A. All experiments were independently performed at least four times, and the blots shown are representative of at least four experiments (n = 4). Bar graphs depict mean fold alterations above/below the controls (± standard deviation).DMSO = dimethyl sulfoxide, Tel = telmisartan, Losa = losartan, Fima = fimasartan, eNOS = endothelial nitric oxide synthase, PP2Ac = protein phosphatase 2A catalytic subunit, NO = nitric oxide, BAEC = bovine aortic endothelial cell.
Differences were considered statistically significant at *P < 0.05 or **P < 0.01.
|
Telmisartan down-regulates p-eNOS-Ser1179 and NO levels and up-regulates PP2Ac expression via a PPARγ-independent pathway
 | Fig. 4Tel decreases p-eNOS-Ser1179 levels and NO production and increases PP2Ac expression via a PPARγ-independent pathway. (A, B) BAECs were co-treated with 20 µM Tel and 5 µM GW9662 (a specific and irreversible PPARγ inhibitor) for 24 hours in the presence of 25 mM D-glucose, and then levels of p-eNOS-Ser1179 and PP2Ac expression were assessed by western blotting, as described in Fig. 2. (C) BAECs were treated as described above, and then NO production was measured as described in Fig. 1A. All experiments were independently performed at least four times, and the blots shown are representative of at least four experiments (n = 4). Bar graphs depict mean fold alterations above/below the controls (± standard deviation).DMSO = dimethyl sulfoxide, Tel = telmisartan, eNOS = endothelial nitric oxide synthase, n.s. = not significant, PP2Ac = protein phosphatase 2A catalytic subunit, NO = nitric oxide, PPARγ = peroxisome proliferator-activated receptor γ, BAEC = bovine aortic endothelial cell.
|
Telmisartan reduces p-eNOS-Ser1179 and serum nitrite levels and increases PP2Ac expression in aorta tissues and sera of HFD-fed mice
 | Fig. 5Tel reduces p-eNOS-Ser1179 and serum NO levels, and enhances PP2Ac expression in the aortas and sera of HFD-fed mice. (A) Schematic diagram of animal experiments. Hyperglycemic mice were prepared by feeding male C57BL/6 mice with an HFD (60% fat kcal) for 13 weeks and then randomized to a vehicle-treated group (n = 6) or a Tel-treated group (n = 6; 5 mg/kg body weight/day), as described in the METHODS. (B, C) After being treated as described above, mice were sacrificed and aortas were dissected. Total aortic proteins were subjected to western blotting to assess p-eNOS-Ser1179 levels and PP2Ac expression (Fig. 2). (D) After mice had been treated as described in Fig. 5A, sera were obtained and nitrite levels were measured as described in the METHODS. The blots shown are representative of at least six aortas from each group. Bar graphs depict mean fold alterations above/below controls (± standard deviation).HFD = high-fat diet, Tel = telmisartan, eNOS = endothelial nitric oxide synthase, PP2Ac = protein phosphatase 2A catalytic subunit, NO = nitric oxide.
Differences were considered statistically significant at *P < 0.05.
|
Telmisartan attenuates ACh-induced aorta relaxation
 | Fig. 6Tel attenuates ACh-induced aortic vessel relaxation. (A, B) Rat thoracic aortas were prepared and subjected to a vessel relaxation assay as described in the METHODS. Endothelium-intact aortic rings were treated with 20 µM Tel or vehicle (DMSO) for 24 hours in the presence of 25 mM D-glucose, precontracted with 0.1 µM PE, and then treated with increasing concentrations of acetylcholine (ACh, 0.001–10 µM). Contractile levels immediately before ACh treatment were considered to be contractions of 100%. Tension curves indicates ACh-induced aortic relaxation in response to 20 µM Tel or vehicle (DMSO). The line graph was plotted using mean ± standard deviation at each point (n = 6). All experiments were independently performed at least six times (n = 6).PE = phenylephrine, ACh = acetylcholine, DMSO = dimethyl sulfoxide, Tel = telmisartan.
Differences were considered statistically significant at *P <0.05 and **P < 0.01.
|
DISCUSSION
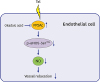 | Fig. 7A schematic illustration of Tel-inhibited NO production and aortic vessel relaxation. Tel, but not losartan or fimasartan, enhances PP2Ac expression in a PPARγ-independent manner in BAECs. PP2Ac up-regulation by Tel reduces p-eNOS-Ser1179 levels and consequently NO production in BAECs and mice. The PP2Ac/p-eNOS-Ser1179/NO signaling pathway regulated by Tel attenuates ACh-induced rat aorta relaxation.Tel = telmisartan, PP2Ac = protein phosphatase 2A catalytic subunit, eNOS = endothelial nitric oxide synthase, NO = nitric oxide, PPARγ = peroxisome proliferator-activated receptor γ, BAEC = bovine aortic endothelial cell, ACh = acetylcholine.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download