Introduction
Peri-implant disease was first introduced in 1987, and was defined as ‘a site-specific infection which yields many features in common with chronic periodontitis.’
1 It was classified into peri-implant mucositis and peri-implantitis in 1992 and has been revised up to date.
2 Nowadays, peri-implant mucositis is considered a state of ‘reversible inflammatory reactions in the soft tissues surrounding a functioning implant’, and peri-implantitis is defined as a state of ‘inflammatory reactions with loss of supporting bone in the tissues surrounding a functioning implant’.
2 Of the two, peri-implantitis is a disease that can severely affect an existing implant.
1
There are 6 main signs and symptoms of peri-implantitis. (1) Bleeding on probing is always present, (2) marginal tissue may be swollen or red, (3) increase in probing depth with loss of attachment, (4) pain in case of severe bone loss, (5) mobility, and (6) loss of bone levels around implants in radiographic evaluation.
13 Due to osseointegration at the apex, loss of bone can be present without signs of mobility, and hence, an implant without mobility does not necessarily mean that it is disease-free.
45 Peri-implantitis can be caused by many factors. Bacterial colonization is mainly the primary cause, and while it is controversial, mechanical overload can also be a contributing factor.
67 History of periodontitis is also known to elevate the risk factors as reported by Karoussis et al.
4; 28.6% of patients with history of chronic periodontitis were affected while only 5.8% were affected in periodontally healthy subjects.
In a recent systematic review, Lee et al.
8 reported a peri-implantitis prevalence of 19.8% at patient level, and 9.3% at implant level. According to this review, almost 20% of patients are at risk of having peri-implantitis, and as the number of patients with dental implants increases, subsequent increase in the incidence of peri-implant infections is inevitable. With the numbers increasing, more attention is drawn to preventing and treating these infections. Most treatment methods are focused on periodontal and surgical means.
9 Meanwhile, preventing such infections is a more multi-disciplinary process, in which prosthodontist plays a major role.
10 In the prosthodontic point of view, one should select materials less prone to bacterial colonization and create them in a cleansable shape. This includes precise marginal fit, cleansable interproximal contours, smooth emergence profile and avoiding over-contoured form.
510 This case report focuses on a successfully rehabilitated peri-implantitis patient, raising awareness of peri-implantitis while discussing the role of prosthodontist in creating a perio-friendly prosthesis.
Case Report
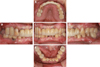
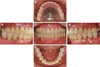
A 69-year old male patient visited the Department of Prosthodontics, Ewha Womans University Mokdong Hospital with a chief complaint of bleeding gums and mobility of teeth (
Fig. 1,
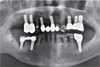
Fig. 2). He had medical history of hyperlipidemia and Alzheimer's disease and was taking medication accordingly. #17, #16, #27, #37, #41, and #47 (FDI numbering system) were missing with four implant FPDs and a single implant crown installed in a private clinic in the United States of America, all between 2007 and 2013. Implants #14, #15, #24, #25, #26, #36, #44, and #46 were ScrewPlant (Implant Direct, Valencia, CA, USA) models and implants #12 and #21 were Legacy Implant (Implant Direct, Valencia, CA, USA) models, all of which are hydroxyapatite-coated implants. Implants #24, #25, #26, #36, and #46 showed full probing depth along with pus discharging from severely swollen gingiva. Implant #15 and #45 also showed a deep probing depth of 7–8 mm. Cantilevered maxillary anterior implant FPD revealed ill-fitting margins with palatal porcelain chipping. Lower anterior teeth, #13, #34, and #35 showed severe mobility. Occlusion was also unfavorable due to generalized attrition, deep overbite, and reverse Curve of Spee. Facial examination along with intraoral inspections showed well-developed masseters, leading to a strong bite force. Generalized chronic periodontitis and localized peri-implantitis along with unfavorable occlusion was diagnosed.
From the observations, four main key points for the treatment were considered. Removal of hopeless teeth and implants, strategic use of remaining implants for the new restoration, remodeling of occlusion, and prevention of attrition due to strong bite force. As the patient wanted new implant prostheses instead of conventional dentures, implant installation after removing the diseased implants were planned. To minimize the risk of recurrent peri-implantitis, attention in designing restorations that are favorable to periodontal tissues was necessary.
A treatment plan was developed after collecting the patient's needs; his first priority was fixed restorations with minimum number of surgeries. However, due to the high economic cost of fixed implants, the patient was willing to try a removable restoration as long as the retention is favorable. Fixed restorations with 6 new implants were planned for the mandible, while an overdenture (OVD) on remaining implants and tooth was planned for the maxilla. The option of fixed implant restorations on the maxilla were ruled-out due to the large defect on the left maxilla, as extensive rehabilitation was expected.
Extraction of all natural teeth except #23, #33, and #43, along with removal of implants #15, #24, #25, #26, #36, #44, and #46 were planned, following the explantation criteria by Lang et al.
5 The remaining implants #12, #14, and #21 on the maxilla were to provide support and retention for the overdenture, along with a metal cap on #23. No additional implants were planned for the maxilla. For the mandible, 6 new implants (implants #32, #34, #36, #42, #44, and #46) would be restored with implant fixed partial dentures (FPDs) with single crowns on #33 and #43. Botulinum toxin injections to reduce masseteric force and selection of materials less prone to attrition were additionally considered.
Diagnostic wax-up and arrangement was performed to provide a blueprint for the restorations (
Fig. 3). During extraction and explantation, alveolar ridge preservation (ARP) was carried out with bovine derived xenograft (Bio-Oss, Geistlich Pharma AG, Wolhusen, Switzerland) and a collagen membrane (Collagen-membrane P, Genoss, Suwon, S. Korea). Although no additional implants were planned on the maxilla, ARP was also performed on the left maxilla due to the extensive crater-like defect. A set of removable prosthesis was then delivered and implant installation was scheduled after 3 months. Cone beam computed tomography (CBCT) was taken with a radiographic stent, followed by planning of the location and size of implants to be installed.
After checking the amount of keratinized mucosa on the mandible, a total of 6 internal conical hex implants (TSIII SA, Osstem, Seoul, Korea) were installed. 5.0 mm diameter implants on both molars, 4.5 mm diameter implants on both premolars, and 3.0 mm diameter implants on both lateral incisors were selected. Guided bone regeneration (GBR) on the left mandible with the same materials as ARP was additionally performed to provide extra bone volume.
During the 3-month healing period, reduction of crown and root canal treatment (RCT) on #23 were carried out. Impression with polyvinyl siloxane (PVS) impression material (ImprintII Garant, 3M ESPE, St. Paul, MN, USA) was taken and a metal cap was fabricated and delivered. After the healing period, second surgery was carried out on the submerged implants. Due to the insufficient amount of crestal bone on #36i, additional GBR was carried out with Bio-Oss and Collagen-membrane P. After waiting another 3-month healing period, second surgery on implant #36 was performed.
Pick-up copings were splinted to each other with acrylic resin (Pattern Resin LS, GC Corporation, Tokyo, Japan) and impression was taken at fixture level with PVS (ImprintII Garant). Bite registration with wax-rim made from baseplate wax (TruWax, Dentsply Sirona, York, PA, USA) and PVS material (Futar-D, Kettenbach GmbH&Co, Eschenburg, Germany) reflecting the removable provisional restoration was taken. Fixed restoration made from acrylic resin (Alike, GC Corporation, Tokyo, Japan) on temporary titanium cylinders (Osstem, Seoul, S. Korea) for the mandible with provisional complete denture on the maxilla were fabricated and delivered (
Fig. 4). Few adjustments were needed before proceeding to the final restorations due to the insufficient interproximal spaces under the FPDs.
Final impressions were taken with conventional methods with splinted pick-up copings (Osstem, Seoul, S. Korea), individual trays (SR Ivolen, Ivoclar Vivadent AG, Liechtenstein), border molding with compound material (Peri Compound, GC Corporation, Tokyo, Japan), and PVS (ImprintII Garant) (
Fig. 5A, 5B). Metal framework and wax-rim with baseplate wax (TruWax) for the maxillary OVD and fixed bite jig on temporary titanium cylinders with pattern resin and baseplate wax were fabricated. Bite registration with PVS (Futar-D) along with face-bow transfer according to the Camper's Plane were conducted, followed by mounting on a semi-adjustable articulator (Hanau articulator, Whip Mix, Louisville, KY, USA) (
Fig. 5C) They were arbitrarily transferred to a virtual articulator on a CAD software (3Shape Dental Designer, 3Shape, Copenhagen, Denmark) to design custom titanium abutments and zirconia restorations (
Fig. 6). The custom titanium abutments were designed to have equi-gingival margins. The zirconia restorations were carefully designed to have cleansable interproximal areas and to avoid excessive contours with emergence profiles as less divergent as possible in order to reduce plaque deposition. After trying in the fabricated custom abutments, final monolithic zirconia crowns and bridges (posteriors: Luxen E2, DentalMax, Cheonan, S. Korea / anteriors: Tanaka EnamelZR, Tanaka Dental, Tokyo, Japan) were milled (Roland DWX-50, Roland DG, Shizuoka-ken, Japan). Gingival shaded porcelain (IPS Gingiva, Ivoclar Vivadent AG, Liechtenstein) was added on the anterior 4-unit bridge for enhanced esthetics. Care was taken to provide sufficient interproximal space between abutment implants and pontics, and to avoid being over-contoured. Precise fitting of margins was verified with the tip of an explorer. Maxillary OVD was conventionally fabricated with bilaterally balanced occlusion (
Fig. 7).
During the treatment process, the patient received a total of 3 botulinum toxin (Botox Inj, Allergan plc, Dublin, Ireland) injections on both masseters. Also, to minimize maxillary denture tooth abrasion from strong masticatory force, zirconia crowns on the denture's posterior teeth were planned. The premolars and molars of the denture were crown prepped and scanned with a digital scanner (3Shape D800, 3Shape, Copenhagen, Denmark). Maxilla and mandible models were mounted on a virtual articulator in the CAD software and two 4-unit splinted zirconia crowns were designed. The articulator simulated bilaterally balanced occlusion. The zirconia crowns were milled with same materials as in the mandible, and were cemented to the denture with dual-cured resin cement (RelyX U200, 3M ESPE, St. Paul, MN, USA) (
Fig. 8). They were additionally adjusted on the actual articulator. Upon trying them in the patient, bite registration (Aluwax, Aluwax Dental Products, Allendale, MI, USA) and clinical remounting was performed. After adjustment, zirconia crowns were cemented with the same dual-cured resin cement and zirconia bridges were cemented to custom abutments with zinc oxide/eugenol cement (Temp-Bond, Kerr, Orange, CA, USA) (
Fig. 9,
Fig. 10). Occlusion was confirmed with T-Scan 8 (Tekscan, South Boston, MA, USA) to check for bilaterally balanced occlusion (
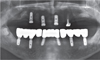
Fig. 11). Overdenture attachments (Locator, Zest Dental Solutions, Carlsbad, CA, USA) were connected to the maxillary implants and OVD with hard relining material (Tokuyama Rebase II, Tokuyama Dental, Tokyo, Japan), two weeks after delivery. The patient expressed satisfaction in both esthetics and function. Patient motivation for oral hygiene control and regular recall intervals were emphasized. Follow-ups to 6 months were carried out without any specific findings, and future periodic follow-ups are planned.
Discussion
Treatment options of peri-implantitis are selected according to the periodontal probing depth (PD), and include mechanical debridement, antiseptic cleansing with chlorhexidine, systematic or local antibiotic therapy, and resective or regenerative surgery.
1112 However, in the case of severe bone loss, even surgical procedures may be inadequate, and explantation should be considered.
5 Lang et al. proposed a criteria for explantation, which are as follows: (1) implant is clinically mobile, (2) cannot be controlled by therapeutic protocols, (3) presence of a suppurative exudate with overt bleeding on probing (BOP) and PD more than 8 mm, (4) perforations of hollow body implants with pain, and (5) radiolucency along the outline of the implant fixture.
5 In this case, implants #24, #25, #26, #36, #44, and #46 met 4 criterions out of 5, and were diagnosed to be hopeless. Meanwhile, implants #14, #12, and #21 did not meet any of the criterions, and was decided to be preserved. This left one implant (implant #15)'s prognosis questionable, since it showed no mobility, no pus discharge, PD of 7 – 8 mm, and radiolucency up to half the length of the implant fixture. When deciding whether to explant the implant or not, surface coating material was taken into consideration. The implants were hydroxyapatite (HA)-coated models, and while HA accelerates implant fixation on new bone tissues, it also has high affinity to bacteria. HA coating that remains longer than required may be susceptible to infection. Hence, once inflammation and progressive bone resorption occurs around the HA-coated implant, recovery is difficult.
131415 Consequently, the questionable implant #15 was decided to be explanted.
Peri-implantitis is known to be induced by surgical risk factors, prosthetic risk factors, and by plaque deposition. Surgical risk factors include malpositioned implants and failed bone reconstruction. Prosthetic risk factors include cement remnants, incorrect margin and contour, presence of loading, abutment unscrewing, and implant fracture.
6 These risk factors sum up to 2 major categories: bacterial colonization and mechanical overload. While bacterial colonization is the main factor, whether mechanical overload induces peri-implantitis is controversial. Naert et al.
7 concluded that overload significantly increase plaque-induced bone resorption in the presence of inflammation. In this case, the patient had unharmonious occlusion such as deep overbite, occlusal interference on lateral excursion, and reverse Curve of Spee. Severe plaque and calculus deposition may have started a plaque-induced resorption, which may have worsened by overload caused by ill-fitted and unharmonious restorations. The main goal in rehabilitating the occlusion was to reduce overjet and overbite, use materials less prone to attrition, achieve bilaterally balanced occlusion, and reduce masticatory force by periodic botulinum toxin injections.
However, even with thorough treatment planning, there were some drawbacks. First of all, the mandible was restored with implant FPDs while the maxilla was restored with a denture, which makes the mandible considerably stronger than the maxilla. While this was inevitable due to the extensive defect and the patient's refusal of additional surgeries, this imbalance may cause excessive force and act as a risk factor to the remaining maxillary implants. Secondly, crown-to-implant ratio of the mandibular FPDs are almost 2:1, posing a risk to the relatively short implants, especially on implant #46. Lastly, the outcome would have been more favorable if there was more keratinized gingiva on the mandible. It is reported that there are associations between peri-implant diseases and the absence of keratinized gingiva, especially when the width is less than 2 mm.
16 In this patient, the keratinized gingival width on mandibular posterior implants were around 2 mm, which could also be an inflammation-inducing factor. Periodic follow-up with occlusal analysis and periodontal evaluation should be executed to minimize these factors.
On the 6th ITI Consensus, it was reported that implants placed in augmented sites display higher variability and lower predictability than those placed in pristine sites in terms of peri-implantitis.
17 This suggests that rehabilitated peri-implant patients may be at a high risk of recurring peri-implantitis. In order to lower the risks, the clinician should follow some protocols. In treatment planning phase, selecting materials for implant fixtures and restorations with less affinity to bacteria should be considered. Zirconia is known to be biologically inert with less plaque accumulation, but more future research is in need.
18 In surgical phase, extensive surgical debridement of inflammatory tissues is essential.
11 Also, in the prosthetic phase, one should give attention to creating hygienic restorations, which should not be over-contoured with interproximal contours made to be cleansable. To avoid over-contoured restorations, the emergence profile starting from the implant fixture to the custom abutment should be taken into consideration. Katafuchi et al.
19 reported that the incidence of periimplantitis increases when the emergence angle, being ‘the angle between the average tangent of the transitional contour relative to the long axis of a tooth, dental implant, or dental implant abutment’, is larger than 30 degrees or when the restoration has a convex profile. According to this investigation, when designing restorations, large emergence angles and convex profiles should be avoided. Also, precise marginal fit is a requisite and supra-gingival margins are preferred when possible.
5 In maintenance phase, instruction in oral hygiene control and patient motivation should be emphasized along with recalling at regular intervals.
5 As a result, the clinician should pay attention in preventing recurrence of periodontal problems with biologically favorable restorations in rehabilitated peri-implantitis patients.









 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download