Abstract
Purpose
The aim of this study was to confirm if Laser-treated implants were soaked in 0.9% NaCl solution for 2 weeks could increase the surface hydrophilicity, and the Remoal Torque of each implant that inserted in rabbit tibia for initial healing period of 10 days.
Materials and methods
Twenty machined titanium surface screws were produced with a diameter 3 mm, length 8 mm. Ten screws had their surface treated with a laser only (laser treated group), and the other 10 were soaked in saline for 2 weeks after surface treatment with a laser (laser treated + saline soaked group). Implants were inserted in rabbit tibia (ten adult New Zealand white rabbits), and the RTQ of each implant was measured after 10 days. The wettability among implants was compared by measuring the contact angle. Surface composition and surface topography were analyzed.
Results
After 10 days, the laser treat + soaking group implants had a significantly higher mean RTQ than the laser treated implants (P = .002, < .05). There were no significant morphological differences between groups, and no remarkable differences were found between the two groups in the SEM analysis.
The stability of an implant can be achieved by complete osseointegration of its surface, and many efforts have been made in this direction.1 Characteristics of the dental implant surface are the primary parameters that affect the rate of osseointegration.23
There are many ways to modify the surface of an implant, such as turning, acid-etching, hydroxyapatite coating, sol-gel coating, sandblasting and acid-etching, grit-blasting, oxidizing, plasma-spray coating, and laser deposition. These methods help to replicate the inherent nature of the bone that promotes the maturation of osteoblasts, increases the contact of the bone and implant, and improves the clinical success rate.4
The laser technique can roughen the surface without requiring direct contact with the implant surface, and therefore, there is no risk of contamination. By using the laser technique, hardness and corrosion resistance are increased, and a surface of high purity with standard roughness and a thick oxide layer is formed. Cho and Jung found that a laser-treated implant had a 2.5 times higher removal torque value (RTQ) compared with a machined implant in an experiment using rabbit tibia.5
When an implant is inserted, bleeding occurs from the bone marrow and peri-implant tissue resulting in the collection of blood around the inserted implant. This leads to the formation of a biofilm, which modulates the host's cellular responses, which progresses to the granulation tissue, followed by immature woven bone.6 The bone formation begins early, during the first week, through the promotion of osteoblast differentiation, production of osteogenic factors.7 Between 1 and 2 weeks, the bone tissue responsible for primary mechanical stability of the device, immediately lateral to the implant region, is resorbed and substituted by newly formed bone.6
The surface energy of the implant, which is related to the concept of wettability, is another surface property for measuring the liquidsolid contact angle (CA) indirectly. The mechanism for imitating the inherent wettability of the bone and its characteristics remains to be determined, but the CA range of implants available on the market is very wide.891011 Many studies have demonstrated improvements in cell adhesion, proliferation, differentiation, and bone mineralization in the early stages using a hydrophilic surface. One study presented increased expression of a differentiation marker in cultured osteoblasts on a hydrophilic surface. In addition, improvement in bone to implant contact at an early stage has been reported in animal and clinical studies.12
Changes in physical and chemical implant surface characteristics affect the hydrophilicity.
Hydrophilic implants show reduced C (Carbon) concentration and increased O2 (Oxygen) concentration. In theory, when the implant surface contacts water, −OH and O2 groups are formed on its outermost layer since the oxide surface is hydrophilic. In several studies, surface morphology, topography, and histomorphometric evaluations, among others, have been performed and indirectly demonstrated that implants with increased hydrophilicity have a reduced healing period.13
Currently, the reduction of the osseointegration time is a topic of particular interest in dentistry.7 It is important to consider that the human body needs a minimum amount of time.14 Early osseointegration provides the immediate loading of dental implants.15
To the best of our knowledge, no studies have measured hydrophilic implant removal torque values (RTQ), a direct measure of osseointegration and healing. Furthermore, several studies have shown that the healing period can be reduced with sandblasted and acid-etched implants; however, there appears to be no studies on the effects of laser-treated implants on healing period using RTQ measurements.13
In this study, laser-treated implants were soaked in 0.9% NaCl solution for 2 weeks to increase the surface hydrophilicity. Implants were inserted in rabbit tibia and the RTQ of each implant was measured. The aim of this study was to clinically interpret the RTQ values obtained, and to demonstrate an increase in the hydrophilicity facilitated osseointegration between the bone and implant surface in the short-term.
Machined-surface titanium grade 23 screws 8 mm long and 3 mm in diameter (n = 10) were prepared and Nd:YAG (Jenoptix Laser Optik) laser-treated. They were then dry-packed similar to the commercially available laser-etched implants (conventional laser-etched implants, CSM Implant, Daegu, Korea).
Laser-etched implants were soaked in 0.9% NaCl solution for 2 weeks (n = 10) for chemical activation. After 2 weeks of soaking, implants were stored in a 0.9% NaCl solution (laser-etched active implants).
Before inserting the implants, scanning electron microscopy (SEM) was performed at 200×, 1,000× and 2,000× magnifications, and samples were examined under Quanta FEG 650 from the FEI Company (Hillsboro, OR, USA) for surface topography.
Surface roughness was measured by 3D-confocal laser scanning microscopy. A 1310-nm laser beam scanned the surface of the specimen at 4 frames per s through a confocal diagram. Images were constructed on the x, y plane using an InGaAs photo diode through a pinhole. Using a piezo scanner and an Olympus confocal microscope (LEXT OLS3000-IR, Olympus, Tokyo, Japan), the plane image at 640 × 480 pixels was observed, and surface roughness was determined by measuring the three-dimensional image at 0.1-micron units to the height of the z-axis.
Twenty machined-surface titanium disks 1 mm long and 10 mm in diameter were fabricated and Nd:YAG (Jenoptix Laser Optik) laser-treated. Ten disks of the sample were soaked in 0.9% NaCl solution for 2 weeks and the static contact angle (CA) was measured by the sessile-drop technique using Universal Goniometer DSA 20E (Kruss Hamburg, Germany).16 The CA of 20 samples were measured.
Ten adult New Zealand white rabbits were used in this study. The experiment was approved by the Animal Care and Use Committee of Kyungpook National University (KNU 2014-0044), Korea. Rabbits weighing an average of 2.8 kg each were used for these experiments. Before the surgical procedure, the animals were anesthetized with intramuscular injections of tiletamine/zolazepam (0.2 mL/kg, Zoletil 50, Virbac Laboratories, Carros, France), and approximately 1 mL of local anesthetic agent (2% lidocaine) was injected into the area undergoing surgery.
After shaving the hair from both the legs of the rabbit for the surgical procedure, iodine and 75% alcohol were used to disinfect the area. An incision was made, and the soft tissue and periosteal layer were elevated to expose the rabbit's tibia. Twenty implants were installed in the tibia of 10 rabbits (2 implants each); on the left tibia in the experimental group and on the right tibia in the control group. No implant penetrated the other side of the outer cortical layer. A drill bit of 3.0-mm diameter was used and the drilling was conducted at 800 rpm under constant irrigation. The implant insertion torque was measured using a digital torque measuring device (MGT-12 digital torque gauge, Mark-10 Corp, New York, NY, USA). Antibiotics (1 mL, Baytril, Bayer, Germany) and a metabolism booster (Catosal, Bayer) were administered as intramuscular injections for a week after surgery. Rabbits were housed in a low-stress environment. Each rabbit was kept in a cage made of stainless steel (SUS) in breeding farm. We used litter to keep the animal warm and comfortable as well as keep the cage clean. We putted the bowl in the cage, watering it and change it twice a day and used a bowl in the cage to feed. The breeding farm was equipped with a cooler and a heater to keep the temperature from being extremely high or low and to adjust the body temperature constantly. Also, we supplied fresh air to the animals and ventilates to remove the odorous substances in the room. We checked condition of rabbit once a day. After the experiment, T-61 was administered 0.5 – 1.0 mL per body weight via the ear vein of the rabbit. At this time, the drug infusion rate should be slower than 0.2 mL/sec. All other rabbits were healthy during the experiment.
Ten rabbits were euthanized after 10 days (1.5 weeks) and the RTQ was then measured.
T61 was administered through the ear vein of the rabbit at a dose of 0.5 – 1.0 mL per body weight, with a slow rate of drug infusion of less than 0.2 mL/sec (No excitable response occurs when administered slowly). The cadavers that were euthanized by administration of T61 were kept in the carcass storage compartment of the animal laboratory at the Kyungpook National University Hospital and collected periodically by a professional company.
The maximum RTQ was measured and specified in Newton centimeters. After euthanasia, the bone was exposed and the RTQ was determine using a digital torque-measuring device.
The program used for statistical analysis is IBM SPSS statistics ver. 20.0 (IBM Co., Armonk, NY, USA) and the measured data were compared using t-test.
The implant surface was examined using a Quanta FEG 650 SEM (FEI Company, Hillsboro, OR, USA) under a 10-mm working distance in a 1 × 105 mbar SEM chamber. The acceleration voltage was 30,000 V and the resolution was 1024 × 943 pixels. Before implant insertion, surfaces were observed at 200×, 1,000× and 2,000× magnifications (Fig. 1). No substantial differences were observed between both experimental and control groups at either magnification.
We measured the surface roughness (Rz) of the solid implant surface at 10 random sites on the implants. The mean Rz of a laser treated implant was 57.869 µm while that of a laser treated and saline soaking implant was 59.108 µm. There was no difference in implant surface roughness between the experimental (59.108 µm) and control (57.869 µm) groups (Fig. 2).
At the time of installation, the mean implant insertion torque of the control and experimental group was 12.6 and 15.5 Ncm, respectively, and there was no statistical difference between values (P > .05, Table 2).
After 10 days, there was a statistically significant difference in mean RTQ between the control and experimental groups (16.73 and 23.12 Ncm, respectively, P < .05, Table 3).
The level of osseointegration can be affected by several factors such as biocompatibility, surface design, implant surface treatment, bone type, surgical technique, and implant loading control during the healing period.17 Over the past few years, implant surface properties including topography and wettability have emerged as an implant research topic. The wettability of an implant surface can be improved by the hydrophilicity of the initial contact between the implant surface and host interface, which allows the formation of protein-rich films resulting in an improvement of interactions between ions and water.
Conventionally, implant surfaces were dry and exposed to air, making them hydrophobic due to the adsorption of −C or −CH groups. A hydrophobic implant surface impedes the adherence of protein molecules to the surface, which interferes with a series of cellular response. This process is important because osseointegration takes time.14 Early osseointegration allows immediate loading, where loading is applied 1 week after insertion of an implant, or early loading, where loading is applied 1 week to 2 months after insertion.15 One of the methods to reduce surface energy is the liquid soaking of implants. Compared with conventional methods, protein adherence to an implant surface and the activity of osteoblasts increase, improving bone production. Previous studies have shown that a hydrophilic surface facilitates gene expression, osteoblast behavior, bone mineralization, and initial osseointegration.1819
The RTQ test is a very effective way to evaluate the degree of osseointegration between an implant and the bone. Ivanoff et al.20 claimed that RTQ was closely related to the amount of the bone in the implant-bone contact and thread. However, since RTQ is based on the shear strength between an implant and the bone, it does not directly indicate the bone response or quantity on the implant surface. If the implant was inserted in the bicortical bone, the RTQ could be high regardless of bone growth.
In the present study, the surfaces of 20 implants were laser-treated. Among them, 10 samples from the experimental group were soaked in 0.9% NaCl solution for 2 weeks to increase wettability. The RTQ was then measured to observe the biological responses of the bone following implant installation for 10 day healing period, RTQ showed the significant difference.
Albrektsson et al. showed that the complete healing process in rabbits takes 6 weeks, whereas healing requires 3 – 4 months in humans.21 From this, 10 days of rabbit is equivalent to 3 – 4 weeks of human and saline soaking procedure enhances the coherence with bone which facilitates early loading.
In the SEM analysis, no remarkable differences were found between the two groups. The control group had a surface roughness of 59.108 µm, while that in the experimental group was 57.869 µm. This implies that there were no significant morphological differences between groups.
However, a significant difference in contact angle (CA) was recorded. The experimental group presented a strong preference to hydrophilicity while the control group showed preference to hydrophobicity. In other words, wettability affects osseointegration in rabbit tibia. In addition, a strong bone response was induced from the hydrophilic surface. A greater amount O2 was observed in the experimental group, which contributed to the formation of titanium oxide layer on the implant's surface.
Implants soaked with 0.9% NaCl solution maintain hydrophilicity and block their surfaces from the air, thus preventing contamination of the −C and −CH groups. This method protects the surface and helps to maintain hydrophilicity so that it increases pre-osteogenic and proangiogenic effects, as well as augments osseointegration in animals and humans.2223
The results obtained in this study are in agreement with those of previous studies that demonstrated the beneficial properties of wettability in the early acceleration of osseointegration in both animals17 and humans.23 But no one reported that there was a significant difference in the short period soaking of 10 days.
Based on the results of this experiment, we may infer that bone healing is attributed to chemical changes, as opposed to micro surface topography.
The aim of this study was to investigate the differences in chemical properties and wettability between two different implant groups; 0.9% NaCl solution soaked laser-treated implant surfaces and non-soaked laser-treated implant surfaces. The two implant groups showed a similar microtopography. An increase in the hydrophilicity in the experimental implant group facilitated osseointegration between the bone and implant surface in the short-term, as demonstrated by RTQ measurements.
Figures and Tables
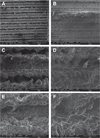 | Fig. 1Scanning electron microscopy of implant surfaces. There is no difference between the control group (B, D and F) and experimental group (A, C and E). A and B (original magnification 200×), C and D (original magnification 1,000×) and E and F (original magnification 2,000×). |
 | Fig. 2Surface roughness. (A) CSM: mean surface roughness (SRz: 100 × 100 µm area): 57.869 µm, (B) CSM saline: mean surface roughness (SRz: 100 × 100 µm area): 59.108 µm.There is no significant difference in surface roughness measurements between the experimental and control groups.
|
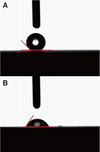 | Fig. 3Contact angle. (A) Control group − contact angle: 134.9∼, (B) Experimental group − contact angle: 50.5∼. |
References
1. Parithimarkalaignan S, Padmanabhan TV. Osseointegration: An update. J Indian Prosthodont Soc. 2013; 13:2–6.

2. Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000; 84:522–534.

3. Wennerberg A, Jimbo R, Stübinger S, Obrecht M, Dard M, Berner S. Nanostructures and hydrophilicity influence osseointegration: a biomechanical study in the rabbit tibia. Clin Oral Implants Res. 2014; 25:1041–1050.

4. Anil S, Anand PS, Alghamdi H, Jansen JA. Dental implant surface enhancement and osseointegration. In Tech. 2011. p. 83–108. http://www.intechopen.com/books/implant-dentistry-a-rapidly-evolving-practice/dental-implant-surface-enhancement-and-osseointegration.
5. Cho SA, Jung SK. A removal torque of the laser-treated titanium implants in rabbit tibia. Biomaterials. 2003; 24:4859–4863.

6. Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003; 14:251–262.

7. Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009; 20:172–184.

8. Sittig C, Textor M, Spencer ND, Wieland M, Vallotton PH. Surface characterization of implant materials c.p. Ti, Ti-6Al-7Nb and Ti-6Al-4V with different pretreatments. J Mater Sci Mater Med. 1999; 10:35–46.
9. Massaro C, Rotolo P, De Riccardis F, Milella E, Napoli A, Wieland M, Textor M, Spencer ND, Brunette DM. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: chemical composition. J Mater Sci Mater Med. 2002; 13:535–548.
10. Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007; 23:844–854.

11. Rupp F, Scheideler L, Eichler M, Geis-Gerstorfer J. Wetting behavior of dental implants. Int J Oral Maxillofac Implants. 2011; 26:1256–1266.
12. Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005; 74:49–58.

13. Sartoretto SC, Alves AT, Resende RF, Calasans-Maia J, Granjeiro JM, Calasans-Maia MD. Early osseointegration driven by the surface chemistry and wettability of dental implants. J Appl Oral Sci. 2015; 23:279–287.

14. Elias CN. Factors affecting the success of dental implants. In : Turkyilmaz I, editor. Implant dentistry: a rapidly evolving practice. Rijeka: InTech;2011. p. 319–364.
15. Weber HP, Morton D, Gallucci GO, Roccuzzo M, Cordaro L, Grutter L. Consensus statements and recommended clinical procedures regarding loading protocols. Int J Oral Maxillofac Implants. 2009; 24:180–183.
16. Kwon JU, Cho SA. Comparison of removal torque of saline-soaking RBM implants and RBM implants in rabbit tibias. J Korean Acad Prosthodont. 2018; 56:1–7.

17. Sela MN, Badihi L, Rosen G, Steinberg D, Kohavi D. Adsorption of human plasma proteins to modified titanium surfaces. Clin Oral Implants Res. 2007; 18:630–638.

18. Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004; 83:529–533.

19. Donos N, Hamlet S, Lang NP, Salvi GE, Huynh-Ba G, Bosshardt DD, Ivanovski S. Gene expression profile of osseointegration of a hydrophilic compared with a hydrophobic microrough implant surface. Clin Oral Implants Res. 2011; 22:365–372.

20. Ivanoff CJ, Sennerby L, Lekholm U. Influence of mono- and bicortical anchorage on the integration of titanium implants. A study in the rabbit tibia. Int J Oral Maxillofac Surg. 1996; 25:229–235.

21. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–170.

22. Textor M, Sittig C, Frauchiger V, Tosatti S, Brunette DM. Properties and biological significance of natural oxide films on titanium and its alloys. In : Brunette DM, Tengvall P, Textor M, Thomsen P, editors. Titanium in medicine: material science, surface science, engineering, biological responses and medical applications. New York: Springer;2001. p. 171–230.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download