This article has been
cited by other articles in ScienceCentral.
Abstract
This is a report of a 58-year-old female with Cushing syndrome who underwent posterior lumbar fusion and lost both her vision completely. She was diagnosed with posterior ischemic optic neuropathy. Cushingoid features such as buffalo hump and central obesity might have attributed in triggering posterior ischemic optic neuropathy. When laid prone for surgery, perioperative high abdominal pressure causes venous hypertension leading to increase amount of blood loss. To compensate, infusion of large quantities of intravenous fluids is necessary which leads to hemodilution which decreases ocular perfusion pressure. Hypercoagulability of Cushing syndrome is also potentially a risk factor of this condition which increases the incidence of venous thromboembolism. For there is no known effective treatment for posterior ischemic optic neuropathy, means to prevent this complication must be strategically reviewed. When performing long spine surgery on patient who has Cushing syndrome or cushingoid features, caution must be taken to avoid this devastating complication.
Go to :

Keywords: Spinal fusion, Cushing syndrome, Posterior ischemic optic neuropathy, Intraocular pressure
INTRODUCTION
Blindness after nonocular surgery is a rare and catastrophic complication. One or more small ciliary arteriole occlusions in the optic nerve are responsible for the infarction.
13) While the pathogenesis of ischemic optic neuropathy is not fully understood, prone position, anemia, hypotension and venous congestion have been suggested as possible risk factors.
679141718) This is a report of a female with iatrogenic Cushing syndrome who lost both her vision after spinal surgery. Informed consent statement was obtained for this study.
Go to :

CASE REPORT
A 58-year-old female underwent spinal fusion surgery for spinal stenosis and scoliosis to correct coronal imbalance on September 13, 2010. She suffered from bilateral leg weakness and aggravated back pain. On magnetic resonance imaging (MRI) scan, multilevel stenosis was found on lumbar spine accompanying scoliosis. To control the symptoms, she went to several hospitals and had frequent steroid injections on her back and knee joints. As a result of numerous injections over a long period of time, she manifested cushingoid features when she came to our institute. She had facial flushing, moon face, buffalo torso, central obesity with thin arms and legs, multiple bruising with thin skin and severe atrophy on her extremities. Her body mass index (BMI) was 22.2 kg/m2. Iatrogenic Cushing syndrome was confirmed after running several tests such as 24-hour urinary free cortisol testing, overnight dexamethasone test and rapid adrenocorticotropic hormone test.
She suffered from weakness which the motor grade for hip flexion were GII. Under the diagnosis of acute bilateral polyradiculopathy of L4-S1 and scoliosis, laminectomy and discectomy of L1/2, L3/4 L4/5, interbody fusion of L3/4 and L4/5 with screw fixation from L1 to L5 was planned (
FIGURE 1).
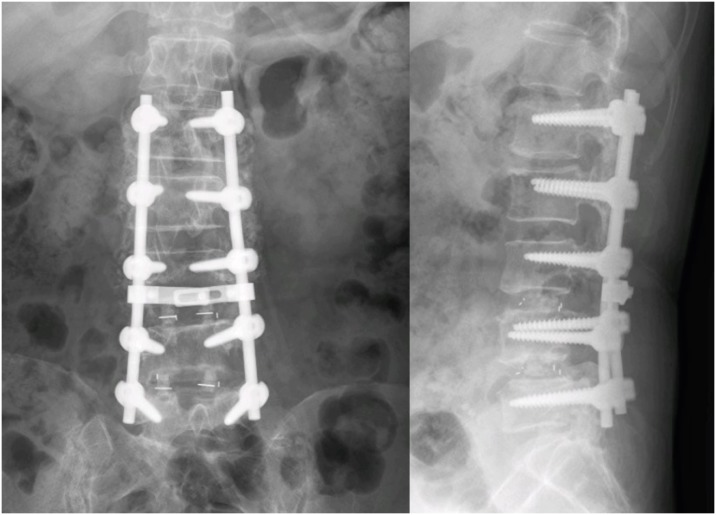 | FIGURE 1 Postoperative X-ray image showing interbody fusion and screw fixation from L1 to L5.
|
On the day of surgery, the patient was allocated in prone position on a spine table placing her head on a soft foam cushions with cutouts for the eyes, nose and mouth. It took 11 hours to complete the operation which was longer than expected. The total amount of blood loss was approximately 13,000 mL and her hemoglobin and haematocrit fell from 13.2 and 40.4 to the lowest of 5.8 and 17 respectively. Intraoperatively, the lowest systolic blood pressure was 70 mmHg which maintained for 5 hours. For fluid resuscitation, 16,000 mL of crystalloid and 1,000 mL of colloid was infused with 16 packs of packed red blood cell and 6 packs of fresh frozen plasma.
Postoperatively she was sent to intensive care unit but didn't complain anything about her eyes. She said she saw everything bright and thought it was because of the ointment applied on her eyes. Not until the fifth day she complained about her bilateral blindness which brought our attention. On seeing the ophthalmologists, her eyelids were swollen for over 5 days but the intraocular pressure was 18 mmHg on her right and 19 mmHg on her left. She had anisocoric pupil, right being bigger than the left with no response to light. Fundoscopic examination showed flat and pale disc without swelling on both eyes (
FIGURE 2). On the orbit MRI, the optic nerve showed high signal intensity on diffuse weighted image, diffusion restriction on apparent diffusion coefficient map and mild diffuse peripheral enhancement along intraorbital segment (
FIGURE 3 &
4). Low dose steroid was administered after surgery for Cushing syndrome but upon the funduscopic finding which was evident of posterior ischemic optic neuropathy it was surmounted to high-dose.
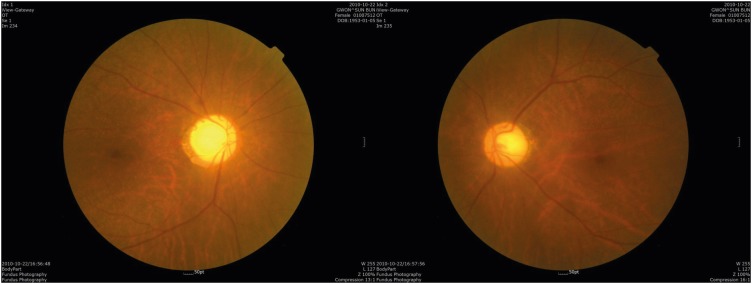 | FIGURE 2 Fundoscopic image showing posterior ischemic optic neuropathy. Both eyes have flat and pale disc without swelling.
|
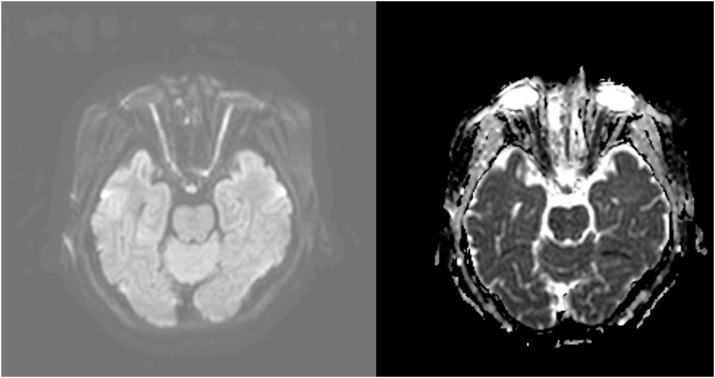 | FIGURE 3 Orbit magnetic resonance imaging showing high signal intensity on the optic nerve on diffuse weighted image and diffusion restriction on apparent diffusion coefficient map.
|
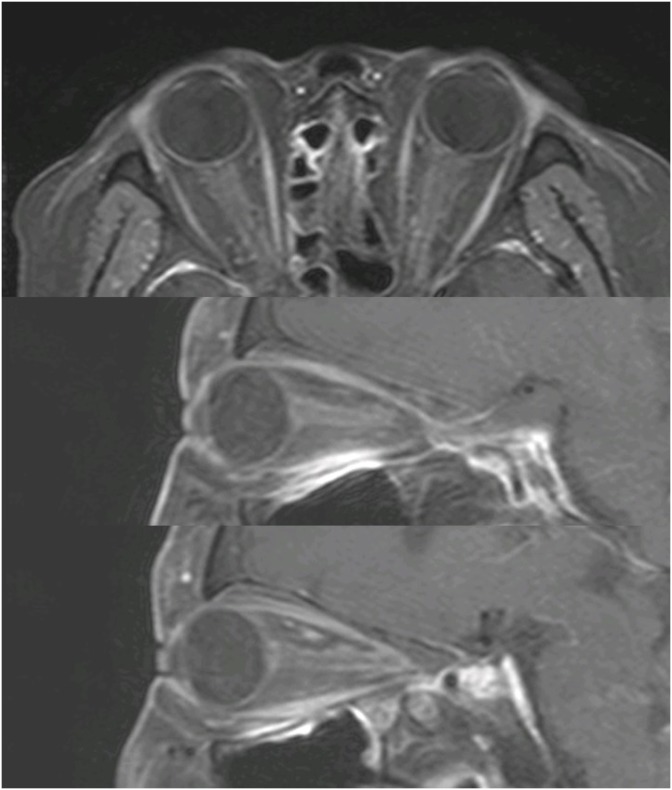 | FIGURE 4 Orbit magnetic resonance imaging showing mild diffuse peripheral enhancement along intraorbital segment.
|
She had recovered light reflex and a small island of vision in inferotemporal quadrant of her left eye after a month. A year after, she was able to discriminate people from objects with the left eye. However, her right eye remained blind.
Go to :

DISCUSSION
The incidence of postoperative visual loss after nonocular surgeries account for 0.028% to 0.2%.
1) There could be several explanation for postoperative visual loss which should be distinguished from central retinal occlusion and cortical blindness. Among them, posterior ischemic optic neuropathy (PION) is known to be most common.
14) Suggestive risk factors of PION include prolonged surgery in the prone position, venous congestion of the head, large blood loss with large fluid resuscitation, hypotension, anemia, use of vasopressors, emboli, and susceptible patient vascular anatomy and physiology.
679141718) Many risk factors fell into the categories in this case mainly due to iatrogenic Cushing syndrome.
Glucocorticoids are frequently used for both diagnostic and therapeutic purposes.
5) It has been more than 60 years since the introduction of glucocorticoid therapy as an effective treatment for patients with inflammatory process. Although glucocorticoid therapy has been widely accepted as an essential part of certain clinical settings, long-term administration can suppress the hypothalamic pituitary-adrenal axis, causing secondary adrenocortical insufficiency with surgical or medical stress.
12) It is impossible to predict the regimen that will suppress the hypothalamic-pituitary-adrenocortical axis and by that increase the risk of developing adrenal insufficiency.
11)
The most common cause of Cushing syndrome is iatrogenic and it is the consequence of long-term glucocorticoid administration using more than 7.5 mg prednisone per day.
2) If the amount of nerve blocks and joint injections with steroids are not counted, patients will be susceptible to this syndrome after steroid abuse. The syndrome itself increases the risk of peri- and postoperative complications.
The most common cause of cushingoid features is iatrogenic corticosteroid use which increases circulating corticosteroid levels leading to Cushing syndrome.
3) Buffalo torso and central obesity could have adversely affected the patient during surgery.
716) Prone position itself raises intraocular pressure which rises significantly with time. This could potentially drop the perfusion pressure of the ciliary arterioles to cortical values.
8) Buffalo torso aggravated head congestion for making it hard to place the head at the level of heart or higher.
BMI was reported to influence intraabdominal pressure in the prone position more than in the supine position during lumbar spinal surgery. A positive correlation of blood loss and intraabdominal pressure and with BMI in the prone position was reported.
1015) Intraocular pressure also rises if there is direct pressure on the abdomen.
8) Placing the patient in prone position causes intraabdominal pressure to increase followed by venous hypertension which leads to intraoperative bleeding. This case eventually resulted in massive bleeding which needed fluid resuscitation.
16) Even though central venous pressure is within normal range, infusion of large quantities of fluids in the head-down position may cause venous congestion in the eyes. Increased venous pressure can also cause increased intraocular pressure within the eyes. It is thought to be one of the main factors responsible for triggering posterior ischemic optic neuropathy in susceptible patients. It creates an environment resembling compartment syndrome at the level of the optic canal which obstructs the arterial blood flow to the optic nerve.
8)
Hypercoagulability is also an issue of Cushing syndrome which increases the incidence of venous thromboembolism. The risk of developing venous thromboembolism has been reported to be 10 times higher in patients with Cushing syndrome. In addition, the risk is even higher after major spine surgery. Based on many studies, increase levels of procoagulant factors especially VIII, IX, and von Willebrand factor, and impaired fibrinolytic capacity leads to hypercoagulable state in Cushing syndrome.
4) Increased production of procoagulant factors with activation of the coagulation cascade shortens activated partial thromboplastin time and an impaired fibrinolytic capacity increases clot lysis time which causes hypercoagulability in Cushing syndrome.
19)
Go to :

CONCLUSION
For there is no known effective treatment for posterior ischemic optic neuropathy, means to prevent this complication must be strategically reviewed.
16) When performing long spine surgery on patient who has Cushing syndrome or cushingoid features caution must be taken to avoid this devastating complication.
Go to :


