This article has been
cited by other articles in ScienceCentral.
Abstract
We encountered a very rare case of spontaneous spinal cerebrospinal fluid (CSF) leakage and a spinal intradural arachnoid cyst (AC) that were diagnosed at different sites in the same patient. These two lesions were thought to have interfered with the disease onset and deterioration. A 30-year-old man presented with sudden neck pain and orthostatic headache. Diplopia, ophthalmic pain, and headache deteriorated. CSF leakage was confirmed in C2 by radioisotope cisternography, and an epidural blood patch was performed. While his symptoms improved gradually, paraparesis suddenly progressed. Thoracolumbar magnetic resonance imaging (MRI) revealed an upper thoracic spinal intradural AC, which was compressing the spinal cord. We removed the outer membrane of the AC and performed fenestration of the inner membrane after T3-4 laminectomy. Postoperative MRI showed complete removal of the AC and normalized lumbar subarachnoid space. All neurological deficits including motor weakness, sensory impairment, and voiding function improved to normal. We present a case of spontaneous spinal CSF leakage and consecutive intracranial hypotension in a patient with a spinal AC. Our report suggests that if spinal CSF leakage and a spinal AC are diagnosed in one patient, even if they are located at different sites, they may affect disease progression and aggravation.
Go to :

Keywords: Cerebrospinal fluid leakage, Intracranial hypotension, Arachnoid cyst
INTRODUCTION
Spontaneous intracranial hypotension caused by leakage of cerebrospinal fluid (CSF), is a major cause of postural headache.
510) Although connective tissue diseases, trauma, and spinal procedure-related injuries are mainly associated with pathogenesis, the pathogenesis of spontaneous CSF leakage remains unclear.
21012)
A normal arachnoid membrane is composed of a single layer of membranes and encircles the brain and spinal cord. When the membrane is divided into two layers and CSF accumulates between the two layers, this causes an arachnoid cyst (AC). Most ACs are congenital lesions in the brain, and rarely occur in the spinal cord. Although spinal ACs may occur secondary to trauma, hemorrhage, surgery, or inflammation, most of them are known to be idiopathic or congenital.
8913) AC may last for a lifetime without any symptoms, may not change the size of the cyst until symptoms appear, or on the rare occasion, disappear spontaneously.
We present a very rare case of spontaneous spinal CSF leakage and spinal intradural AC that were diagnosed at different sites in the same patient, and suggest the relation to their etiology, onset, and deterioration.
Go to :

CASE REPORT
A 30-year-old man presented with acute neck pain and orthostatic headache during exercise. Initial brain CT performed the next day showed normal findings. Four days later, he had diplopia, ophthalmic pain, and a more severe headache. Aside from a mild contrast enhancement of meninges, no remarkable observations such as leakage of CSF were noted on magnetic resonance imaging (MRI) (
FIGURE 1).
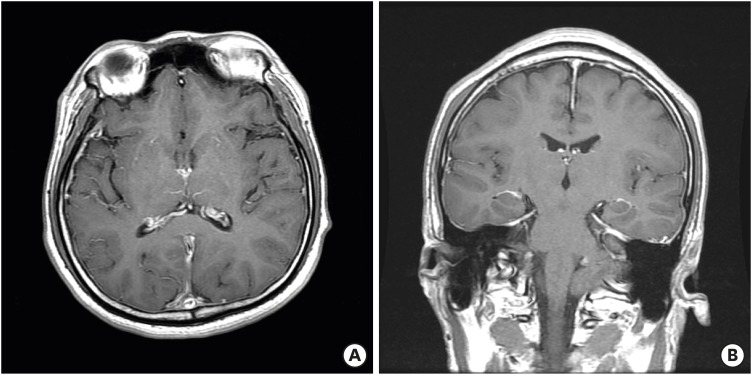 | FIGURE 1 Magnetic resonance images showed mild contrast enhancement of meninges.
|
We performed radioisotope (RI) cisternography with the suspicion of intracranial hypotension as a result of CSF leakage. As RI cisternography revealed CSF leakage at the C2 level (
FIGURE 2), we performed an epidural blood patch through the interlaminar space of C2-3. Five days after the epidural blood patch, the patient experienced weakness in both legs and sensory loss below the T10 dermatome, and dysuria suddenly occurred. We performed thoracolumbar MRI and observed mild expansion of the subarachnoid space at the upper thoracic levels and contrast enhancement of the thoracolumbar arachnoid membrane (
FIGURE 3A & B). In contrast, the lumbar subarachnoid space became narrow (
FIGURE 3C & D). In the CSF examination, xanthochromia was observed and pressure was elevated up to 34 mmHg. White blood cell count was 510 (mm
3; mono, 90%), total protein level was 213.6 mg/dL, and glucose level was 51.3 mg/dL. We thought he developed chemical arachnoiditis; thus, we administered methyl prednisolone intravenously for 17 days. However, symptoms and manifestations did not improve. We performed MRI again and could find an intradural AC severely compressing the spinal cord at the upper thoracic spine (
FIGURE 4).
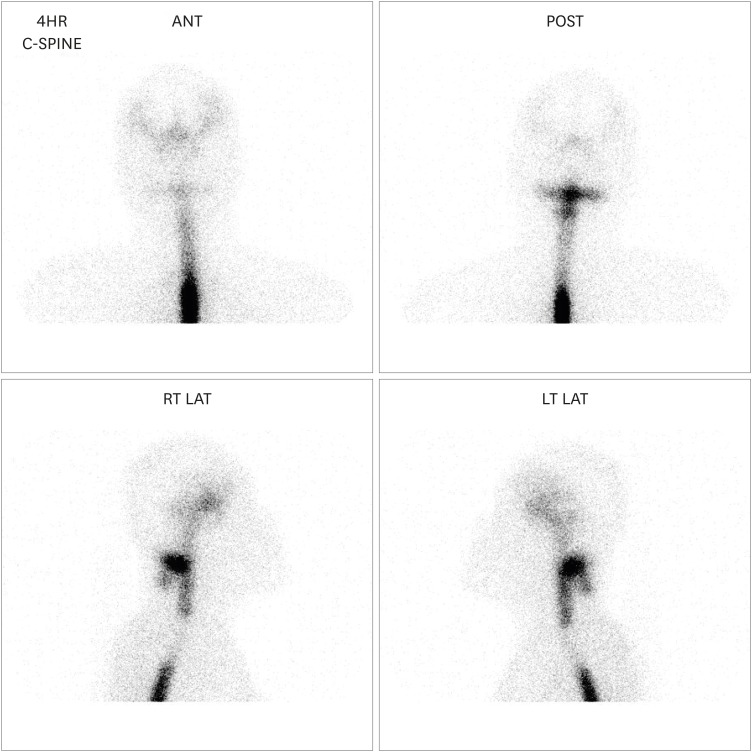 | FIGURE 2
Radioisotope cisternography revealed cerebrospinal fluid leakage at the C2 level.
ANT: anterior, POST: posterior, RT LAT: right lateral, LT LAT: left lateral.

|
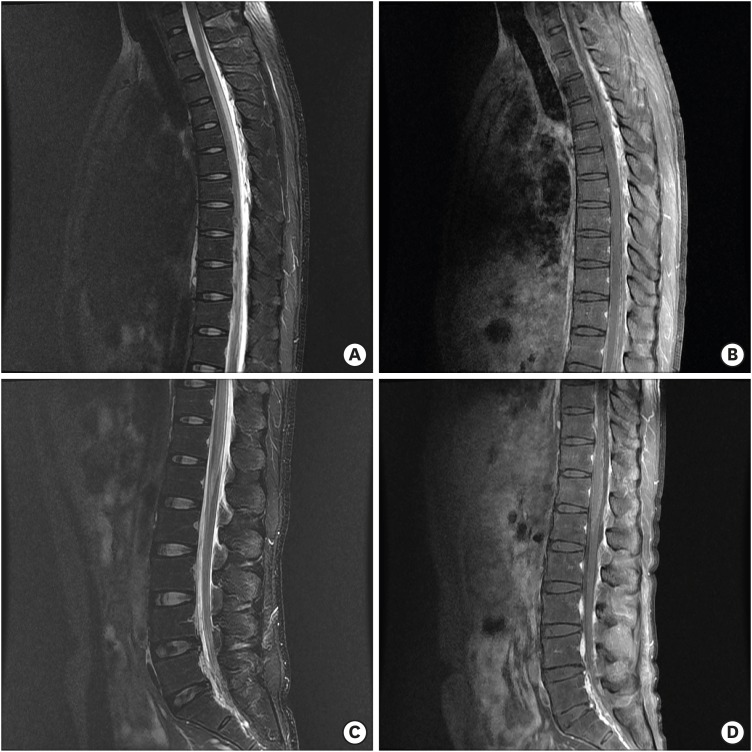 | FIGURE 3 Thoracolumbar magnetic resonance imaging showed mild expansion of the subarachnoid space at the upper thoracic levels with contrast enhancement of the arachnoid membrane (A, B). In contrast, the lumbar subarachnoid space became narrow (C, D).
|
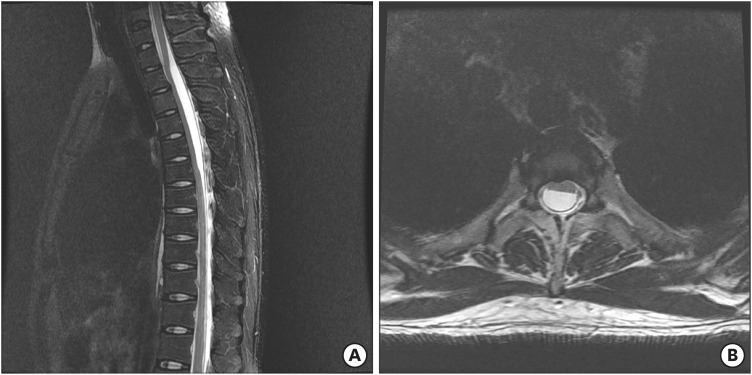 | FIGURE 4 Magnetic resonance imaging revealed an intradural arachnoid cyst severely compressing the spinal cord at the upper thoracic spine.
|
Prior to performing surgery, neurological examination revealed grade IV motor impairment on the right side, grade III motor impairment on the left side, sensory impairment at the lower level of T10 and voiding difficulty. T3-4 laminectomy was performed with the patient in the prone position under general anesthesia. No abnormality such as malformation or tumor was observed in the epidural space. When the dura mater was incised, AC was observed, but no other abnormality was observed in the intradural space.
We peeled off and removed the outer membrane of the AC and made fenestration through the inner membrane of AC. Postoperative MRI showed that AC was removed completely, compression of the spinal cord disappeared, and the subarachnoid space at the lumbar levels was normalized (
FIGURE 5). All neurologic deficits including motor weakness, sensory impairment, and voiding function were gradually improved and normalized.
 | FIGURE 5 Postoperative magnetic resonance imaging showed that arachnoid cyst was removed completely, compression of the spinal cord disappeared, and the subarachnoid space at the lumbar levels was normalized.
|
Go to :

DISCUSSION
Spontaneous intracranial hypotension is a disease in which intracranial pressure decreases without a history of lumbar puncture, trauma, surgery, or related medical conditions, largely caused by spontaneous CSF leakage in the spine. The cause of spontaneous CSF leakage in the spine has not been identified; however, it is speculated that structural weaknesses in the spinal meninges may exist.
210) In the cases reported so far, it is thought that possible causes include genetic diseases with soft tissue abnormalities such as Marfan syndrome, Ehlers-Danlos syndrome, and meningeal defects caused by osteophytes or degenerative intervertebral disc diseases.
5671014) However, there are many cases that do not reveal the etiologies. The patient described herein did not have any preexisting diseases, but just after diagnosis and treatment of spontaneous CSF leakage, paraplegia abruptly developed and consequently, AC was detected at the thoracic spine. There has been few reports of CSF leakage due to spontaneous or post-traumatic rupture of the spinal extradural AC and consequently intracranial hypotension.
11) However, we think this is the first report that describes intracranial hypotension resulting from spontaneous spinal CSF leakage, followed by abrupt expansion of the intradural spinal AC at a different site. Since both spontaneous CSF leakage and AC are caused by structural defects of the meninges, we might speculate that the etiology of both diseases may be related to each other. Moreover, we hypothesize that these two diseases did not occur simultaneously incidentally, but they acted as a cause of onset and deterioration to each other. Even though we overlooked some findings of RI cisternography performed at the early stage, it already revealed a filling defect region in the upper thoracic spine (
FIGURE 2). In other words, the spinal AC already existed before spinal CSF leakage occurred. We assumed that the spinal AC might partially obstruct the CSF circulation, increasing CSF pressure and possibly leading to CSF leakage at the cervical spine—although it might already be structurally vulnerable.
In many cases, AC does not increase in size. The mechanisms involved in the increase in AC size are as follows. First, the activation of CSF secretion of the inner wall of the AC. Second, differences in osmotic pressure between the subarachnoid space and AC, which have a high protein concentration, cause an influx of CSF into the AC. Third, the inflow of CSF onto the AC via the ball-valve action. Fourth, the increase of intraspinal CSF pressure could stimulate the movement of CSF into the cyst.
134)
In the author's case, several causes can be inferred. Our first hypothesis is that the acute increase immediately after the acute decrease of CSF pressure resulted from leakage and clogging of CSF and consecutive compression of the thecal sac by epidural patch-induced CSF inflow into the AC. The second is the possibility that the radioisotope injected into the subarachnoid space during RI cisternography remained inside the AC and increased osmotic pressure, and consecutively CSF flowed into the AC. The third is that chemical arachnoiditis-induced arachnoid adhesion, consecutive failure of CSF circulation and CSF flux into the cyst.
Although it is unclear what the actual cause of the cyst expansion is, it is certain that the AC gradually grew during the treatment of spinal CSF leakage.
We speculate that congenital thoracic spinal ACs partially obstructed the CSF circulation, elevated the CSF pressure, and induced spontaneous cervical spinal CSF leakage, causing intracranial hypotension. We also propose that by closure of the CSF leakage by the epidural blood patch, chemical arachnoiditis may induce a gradual expansion of the spinal AC by increasing CSF inflow into the AC.
Go to :

CONCLUSION
We present a case of spontaneous cervical spinal CSF leakage accompanied by consecutive intracranial hypotension in a patient with thoracic spinal AC. It is important to note that both diseases can co-occur because both are caused by structural defects of the meninges. Therefore, care should be taken to treat both diseases because they can affect the progression and deterioration of each other even if they are located at different sites.
Go to :


