This article has been
cited by other articles in ScienceCentral.
Abstract
Proton pump inhibitors (PPIs) are widely used for the treatment of gastrointestinal diseases. Incidence of drug hypersensitivity reactions (DHRs) to PPIs has been rising, presumably because of their increased consumption. Most DHR are IgE-mediated, with half of the reactions being anaphylactic. We describe the case of a Caucasian 50-year-old female patient referred to our allergy department after 2 episodes of anaphylaxis. The allergy work-up distinguished PPI as a cause of delayed onset (14 hours) and immediate onset (45 minutes) IgE-mediated DHR.
Keywords: Anaphylaxis, Hypersensivity, Delayed, Drug hypersensitivity, Proton pump inhibitors, Skin tests
INTRODUCTION
Proton pump inhibitors (PPIs) are widely used and the incidence of drug hypersensitivity reactions (DHR) to PPIs has been rising, presumably because of their increased consumption.
Most DHR are IgE-mediated, with half of them being anaphylactic [
1].
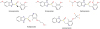
Cross-reactivity within the PPI family (based on skin test [ST] results) is likely related to their similar chemical structure (
Fig. 1). Cross-reactivity to all PPI has already been described [
23].
Fig. 1
Chemical structure of the different proton pump inhibitors: they have a benzimidazole ring and a pyridine ring. Omeprazole, esomeprazole, and pantoprazole have changes in their benzimidazole rings whereas lansoprazole and rabeprazole have modifications in their pyridine ring [3].
CASE REPORT
We received in consultation a Caucasian 50-year-old female patient with previous diagnosis of osteoarthritis and thyroid nodules under surveillance. She denied any regular daily medication or history of atopic disease.
The patient was referred to our allergy department after 2 episodes of anaphylaxis.
In the first episode, the patient took a 20-mg omeprazole capsule without intercurrences. The next morning, she took a 400-mg ibuprofen tablet, without any other concomitant drug, namely omeprazole.
Three hours after taking ibuprofen and 14 hours after taking the IPP, she experienced intense abdominal pain and ocular burning sensation, followed in minutes by lip angioedema, dyspnoea and lipothymia. She was admitted to the Emergency Department (ED) and treated with intramuscular adrenaline with complete recovery after 24 hours. Her serum tryptase level was elevated during the episode (35.4 ng/mL) and returned to normal after recovery (3.4 ng/mL). Prior to this episode, the patient had taken omeprazole and ibuprofen multiple times without intercurrences. During her ED visit, she was told that it was likely that the ibuprofen was the culprit, based on chronology details.
Six months later, about 45 minutes after taking a 40-mg pantoprazole capsule, the patient developed severe pelvic pain, diffuse erythema and palmar-plantar pruritus. She took oral H1 antihistamines and corticoid medication with improvement within hours. The patient denied ingestion of suspected foods or other drugs simultaneously, specifically nonsteroidal anti-inflammatory drugs (NSAID). After these two reactions, the patient did not have any PPI or NSAID, except diclofenac, which she tolerated.
Based on her clinical history we suspected PPIs, but the implication of ibuprofen could not be ruled out for the first episode. Skin prick tests with solutions of non-irritating concentrations [
4] of omeprazole (40 mg/mL), esomeprazole (40 mg/mL), lansoprazole (30 mg/mL), pantoprazole (40 mg/mL), and rabeprazole (20 mg/mL) were performed. They were all positive after 30 minutes, except for lansoprazole (
Table 1).
Table 1
Mean wheal diameter of skin-prick tests with proton pump inhibitors and with histamine di-hydrochloride

|
Tested element |
Mean wheal diameter (mm) |
|
Diluent, chlorine saline |
0 |
|
Histamine (10 mg/mL) |
10 |
|
Omeprazole (40 mg/mL) |
7 |
|
Esomeprazole (40 mg/mL) |
6 |
|
Lansoprazole (30 mg/mL) |
0 |
|
Pantoprazole (40 mg/mL) |
4 |
|
Rabeprazole (20 mg/mL) |
9 |
Since lansoprazole only exists in oral formulation that does not allow intradermal tests, a drug provocation test (DPT) was performed on the same day with this alternative PPI and was negative at the cumulative dose of 30 mg. Omeprazole and pantoprazole were thus assumed as the culprit drugs of the reactions. A DPT to ibuprofen was negative. The patient gave her written consent for anonymized data to be used for research purposes.
DISCUSSION
Several features make this case peculiar.
First, the delayed onset of the first reaction: the time course is atypical for an IgE-mediated DHR because her first reaction occurred approximately 14 hours after the drug intake. Perhaps the simplest and most obvious scenario would be to assume that the history provided by the patient was not accurate and that, together with the NSAID, omeprazole had been taken again on the morning of the first episode. The patient has repeatedly denied this hypothesis. Even if this scenario was real, onset of an IgE-mediated reaction on the second intake of a drug is rare [
5].
Assuming that the patient's history is correct, we are facing a case of an anaphylactic reaction, with late onset. Delayed onset of anaphylaxis with food has been described, the same picture is not as well documented in drug allergy. In the case of PPIs, delayed onset of DHR has been described up to four hours after intake [
6].
Several other explanations may be considered:
(1) The serum half-life (t1/2) of omeprazole is of 60 minutes, and it is generally considered that by 7t1/2, the drug has been eliminated [7]. Pantoprazole has a similar pharmacokinetics. However, reactions beyond 7t1/2 of the drug have already been reported for beta-lactams [8], possibly related to the unknown t1/2 of haptenized breakdown derivatives.
(2) The metabolism of PPIs involves 2 cytochrome P450 isoenzymes—CYP2C19 and CYP3A4—with patients having different rates of metabolism and clearance given the gene polymorphisms of these enzymes (poor or extensive metabolizers) [9].
(3) A decreased gastrointestinal permeability (even though an obvious cause was not found) and therefore delayed absorption of omeprazole has been suggested for other similar episodes with other drugs [10].
(4) Intervention of other neurotransmitters and mediators other than those released in immune IgE-mediated hypersensitivity [10] but our patient did have positive ST to different PPIs which is consistent with an IgE-mediated reaction.
(5) The absorption of most oral PPIs is delayed by the enteric coating, applied in the medication to protect the acid-labile PPI from degradation in the stomach [11].
Moreover, NSAIDS may act as a cofactor, increasing its severity. Laguna et al. [
1] reported that in patients who took omeprazole together with NSAIDs, 81% suffered anaphylaxis versus 55% of patients taking omeprazole alone or with other drugs. This hypothesis still does not address the issue of the delayed response during the first episode, after the omeprazole intake, but it suggests that NSAID intake may have been related with the greater severity of the first reaction. Usually patients react similarly to the same drug, both in terms of time onset and severity, however faster subsequent reactions may occur [
8].
Finally, the patient reacted with omeprazole and pantoprazole, drugs belonging to the same pattern of cross-reactivity. Positive ST to one PPI does not anticipate sensitization to another PPI based on theoretical cross-reactivity patterns only, so lansoprazole was tested by DPT, despite rabeprazole positivity in ST.
PPIs are usually not thought to induce anaphylaxis by general practitioners, and even less, delayed anaphylaxis. In emergency conditions, given the delayed reaction onset, some DHR may be attributed to other drugs, exposing the patient to the risk of subsequent intake and renewal of iatrogeny. When faced with severe DHR, all potential elicitors, even those with the most intriguing chronology, must be taken into account and tested.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download